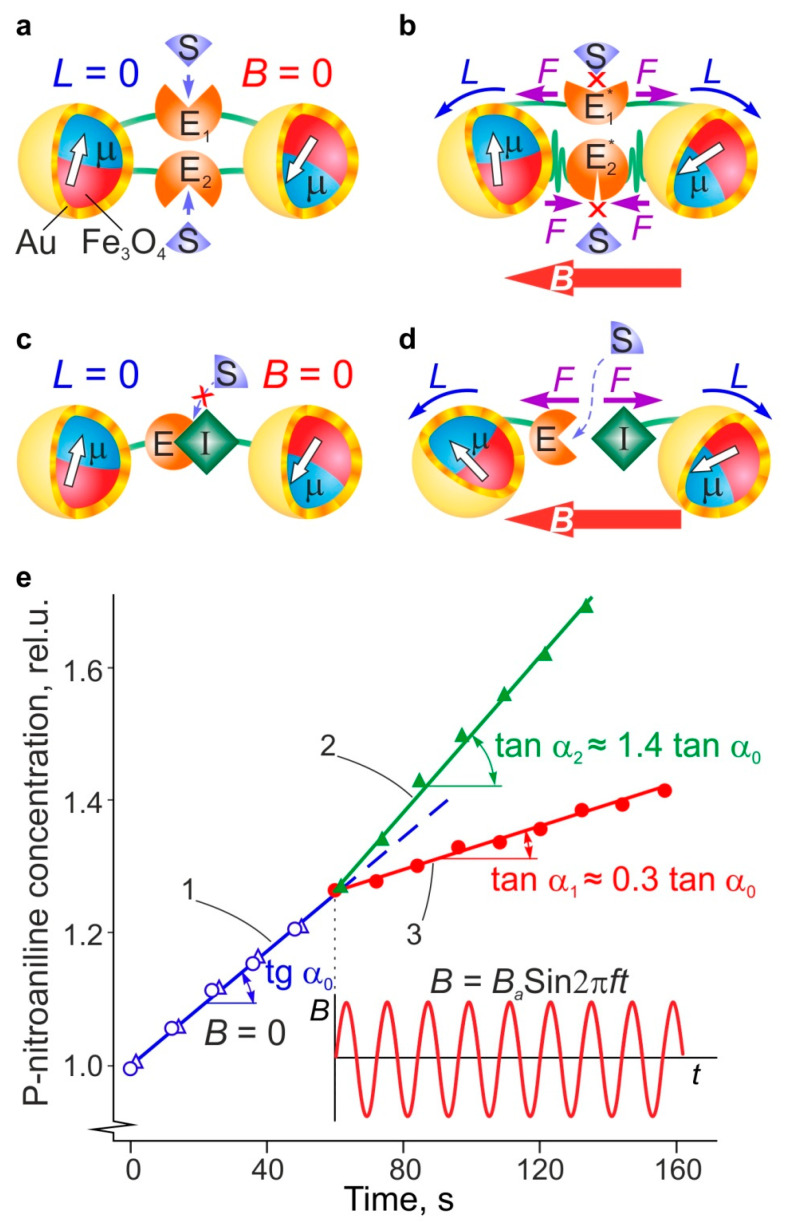
Figure 4.
Effect of a LF MF (B = 88 mT, f = 60 Hz) on the catalytic activity of chymotrypsin macromolecules immobilized in the dimer complex of two MNP: (a) diagram of dimer complex without MFs and (b) upon exposure to a MF; (c) diagram of the f-MNP-ChT-TI-MNP complex without a MF and (d) upon exposure to a MF (E is enzyme, S is substrate, I is inhibitor, TI is Trypsin inhibitor, L is the torque applied to MNPs in LF MF, and F are forces acting on macromolecules in LF MF); (e) the kinetics of the light absorption growth by the product formation during the biocatalytic reaction before the switching on of LF MF (the dependence (1)) and during exposure to the field (dependences (2) and (3) referring to the complexes in (d) and (b), respectively).