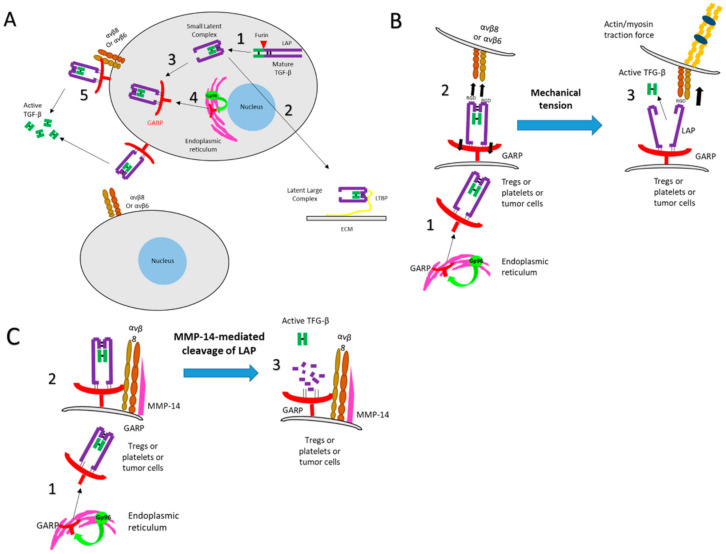
Figure 2.
Multistep process leading to the release of biologically active TGF-β. (A) Latent TGF-β (LTGF-β). TGF-β is synthesized as a homodimeric precursor, cleaved by furin in the Golgi apparatus into LAP and the mature TGF-β, which remain noncovalently associated, as the latent TGF-β (LTGF-β, also called small latent complex) (1). This complex can associate with either the LTGF-β-binding protein (LTBP) into a large latent complex (LLC) that, in turn, associates with the extracellular matrix (ECM) (2), or GARP (3), which allows its membrane expression. GARP requires the ER chaperone Gp96 for its folding and surface expression (4). GARP orients the small latent complex (SLC) for binding and release by integrin αVβ8 or αVβ6 (5). (B) Activation of TGF-β through a protease-independent manner. After the SLC-GARP complex is expressed at the cell membrane with the aid of Gp96 (1), integrins bind to LTGF-β through the RGD motif on LAP (2). Cell contraction or mechanical tension induces a deformation of the surface LAP, mediating the release of the mature form of TGF-β (3). (C) Activation of TGF-β through a protease-dependent manner. After the SLC-GARP complex is expressed at the cell membrane with the aid of Gp96 (1), integrins recruit metalloproteinases (such as MMP-14) or serine proteases by an autocrine or paracrine pathway (2) which cleave LAP thus allowing TGF-β release (3).