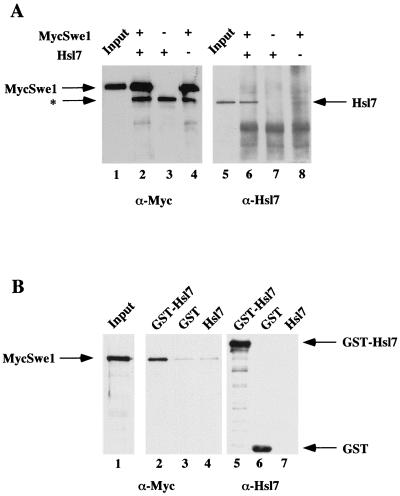
FIG. 4.
Hsl7 physically associates with Swe1 in cell extracts. (A) Extracts of protease-deficient cells expressing either MycSwe1 or Hsl7 were prepared. Equivalent amounts of total protein from these extracts were incubated either alone or together, as indicated, and then subjected to immunoprecipitation using anti-c-Myc MAb 9E10. The resulting immunoprecipitates were resolved by SDS-PAGE and analyzed by immunoblotting either with MAb 9E10 to detect MycSwe1 (left) or with purified rabbit polyclonal anti-Hsl7 antibodies (right). Samples representing ∼1% of the extracts added to each incubation (Input) were examined on the same gels. The asterisk denotes a species present in mouse ascites fluid that adsorbs to the A/G-agarose beads used for immunoprecipitation and nonspecifically cross-reacts with the horseradish peroxidase-linked goat anti-mouse immunoglobulin used for detection of the primary antibody (MAb 9E10). (B) Extracts of protease-deficient cells expressing GST, GST-Hsl, or Hsl7 were prepared. Equivalent amounts of total protein from these extracts were mixed with identical amounts of an extract containing MycSwe1 and then incubated with glutathione-agarose beads. After washing the beads, bound proteins were resolved by SDS-PAGE and analyzed by immunoblotting with mouse anti-Hsl7 antibodies (which were raised against an GST-Hsl7 fusion) to demonstrate specific binding of GST and GST-Hsl7 to the beads (right) and with MAb 9E10 to detect the presence of MycSwe1 (middle). A sample representing ∼1% of the MycSwe1-containing extract added to each incubation (Input) was examined on the same gel (left).