Abstract
In this study, the potential of Carissa carandas Linn. as a natural anti-aging, antioxidant, and skin whitening agent was studied. Various parts of C. carandas, including fruit, leaf, seed, and pulp were sequentially extracted by maceration using n-hexane, ethyl acetate, and ethanol, respectively. High-performance liquid chromatography, Folin–Ciocalteu, and Dowd method were used to investigate their chemical compositions. The inhibitory activities of oxidation process, matrix metalloproteinases (MMPs), elastase, hyaluronidase, and tyrosinase were analyzed. Cytotoxicity was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5 diphenyl tetrazolium bromide assay in a human epidermal keratinocyte line (HaCaT). The results exhibited that ethyl acetate could extract the most ursolic acid from C. carandas, while ethanol could extract the most phenolics and flavonoids. The leaf extract had the highest content of ursolic acid, phenolics, and flavonoids. The leaf extracted with ethyl acetate (AL) had the highest ursolic acid content (411.8 mg/g extract) and inhibited MMP-1, NF-kappa B, and tyrosinase activity the most. Ursolic acid has been proposed as a key component in these biological activities. Although several C. carandas extracts are beneficial to human skin, AL has been proposed for use in cosmetics and cosmeceuticals due to its superior anti-wrinkle, anti-inflammation, and whitening properties.
Keywords: Carissa carandas, ursolic acid, antioxidant, anti-tyrosinase, matrix metalloproteinases, nuclear factor-kappa B, interleukin, human epidermal keratinocyte line
1. Introduction
Carissa carandas Linn. is a fruit-bearing plant that grows as a tiny shrub in the Apocynaceae family that is widely distributed in subtropical and tropical regions and has been used for centuries as a medicinal herb in the Ayurvedic, Unani, and Homeopathic systems [1]. In ethnomedicine, different parts of C. carandas have been used to treat anorexia, asthma, brain disease, constipation, cough, diarrhea, epilepsy, fever, leprosy, malaria, myopathic spams, pain, pharyngitis, scabies, and seizures [1]. C. carandas has been observed to possess a wide spectrum of phytochemical constituents that varied in each part of the plant and result in various biological activities. Tannin, steroidal glycosides, phenolic compounds, and triterpenoidal constituents are abundant in the leaves [2,3]. Several volatile compounds, as well as carissone and carindone, are present in the fruits [1] while the seeds contain fatty acids such as linoleic acid, oleic acid, palmitic acid, and stearic acid [4]. Therefore, all parts of C. carandas would be beneficial for human health and would have a potential to be used for anti-skin-aging. Since C. carandas contained a wide range of phytochemical constituents, from the lipophilic (e.g., fatty acids) to the hydrophilic (e.g., phenolic compounds), the biological activities of the extracts would vary depending on the polarity of the solvents used in the extraction process.
The methanolic extracts of C. carandas leaves have been reported for a potent inhibition potential on DNA damage, oxidation, and inflammatory process [3]. Aside from the leaves, the fruits of C. carandas displayed strong anti-inflammatory activity in rats with carrageenan-induced hind paw edema [5]. The 23-hydroxy ursolic acid, a potent anti-inflammatory agent found in C. carandas, strongly inhibited the production of reactive oxygen species (ROS) from human whole blood phagocytes [6]. Since ROS is implicated with both intrinsic and extrinsic factors, it is thought to play a crucial role in human skin aging [7]. ROS is a crucial factor in the transcription factors activation that result in the release of pro-inflammatory cytokines and the development of matrix metalloproteinase (MPP), inducing cellular senescence and aging in both the epidermis and dermis layers [8]. Furthermore, ROS directly degraded hyaluronan and activated tyrosinase, resulting in symptoms of aging such as coarseness, sallow discoloration, telangiectasia, wrinkling, and abnormal pigmentation, as well as a host of benign, malignant neoplasms, and premalignant [9].
Prevention of oxidative stress, inflammation, and extracellular matrix (ECM) remodeling were purposed as the strategies for anti-skin-aging [7]. Regarding a variety of phytoconstituents of C. carandas, the hypothesis of the study is that C. carandas extract has health beneficial effects and the potential to be used as a natural anti-skin aging ingredient. However, the anti-skin-aging properties of C. carandas, especially in terms of matrix metalloproteinases (MMPs), elastase, and hyaluronidase inhibition, have been rarely investigated. Therefore, the present study was the first to reveal the anti-skin-aging ability of C. carandas extracts. Additionally, the chemical compositions, cytotoxicity effect on human skin cells, antioxidant, anti-inflammation, and anti-tyrosinase activities of C. carandas extracts were also reported.
2. Materials and Methods
2.1. Plant Materials
Leaves and ripened fruits of C. carandas were collected from Samut Songkhram Province, Thailand during May 2017. All plant materials were identified and authenticated by Ms. Wannaree Charoensup, a botanist at the Herbarium of Faculty of Pharmacy, Chiang Mai University. A voucher specimen number 0023269 of C. carandas has been deposited in an Herbarium, Department of Pharmaceutical Science, Faculty of Pharmacy, Chiang Mai University. Seed and pulp of C. carandas were separated from the fruits. Each part of C. carandas, including leaf, seed, and pulp were sliced into small pieces and dried in a hot air oven (UF110, Memert, Germany) set at 50 °C until dryness. The dried C. carandas materials were then ground into a fine powder with a Moulinex mixer blender (Moulinex DB81 blender, Paris, France).
2.2. Chemical Materials
Ursolic acid, hyaluronidase from bovine testis (E.C. 3.2.1.3.5), MMP-1 from Clostridium histolyticum (ChC-E.C. 3.4.23.3), porcine pancreatic elastase (E.C. 3.4.21.36), N-[3-(2-furyl) acryloyl]-Leu-Gly-Pro-Ala, N-succinyl-Ala-Ala-Ala-p-nitroanilide, octylphenol ethoxylate (Triton X-100), alcian blue 8GX, hyaluronic acid, sodium phosphate, sodium chloride, glycerol, and sodium acetate were purchased from Sigma-Aldrich (St. Louis, MO, USA). Protein markers, 30% acrylamide/bis solution, 1 M Tris-HCl, pH 8 solution, and 0.5 M Tris-HCl, pH 6.8 solution were purchased from Bio-Rad Laboratories (Richmond, CA, USA). Sodium dodecyl sulfate (SDS) and bromophenol blue were purchased from Merck (Darmstadt, Germany). Ethanol, hydrochloric acid, ethyl acetate, methanol, n-hexane, acetic acid, and dimethyl sulfoxide (DMSO) were purchased from Labscan (Dublin, Ireland). HPLC grade ethanol was purchased from Carlo Erba Reagents (Cornaredo, Italy). Folin–Ciocalteu solution was purchased from Sigma-Aldrich (St. Louis, MO, USA). Gallic acid, quercetin, Trolox, 2,20-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS), 1,1-diphenyl-2-picrylhydrazyl (DPPH), 2,4,6-tri (2-pyridyl)-s-triazine (TPTZ) were purchased from Sigma-Aldrich (St. Louis, MO, USA), sodium carbonate, ferric chloride, ferrous sulfate, sodium acetate, aluminum trichloride, and potassium persulfate were purchased from Merck (Darmstadt, Germany). 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Sigma-Aldrich (St. Louis, MO, USA). RPMI-1640 medium, Dulbecco’s modified Eagle’s medium (DMEM), and penicillin-streptomycin were purchased from GIBCO Invitrogen (Grand Island, NY, USA). Fetal bovine serum (FBS) was obtained from Biochrom AG (Berlin, Germany). ELISA kits were purchased from eBioscience (San Diego, CA, USA).
2.3. Preparation of C. carandas Extracts
Fine powder of C. carandas fruit, leaf, seed, and pulp were sequentially macerated under continuous stirring by magnetic stirrer (multi-position magnetic stirrer with heater AM4, EOS Scientific Co. Ltd., Bangkok, Thailand) for 24 h at room temperature with n-hexane (3 cycles), ethyl acetate (3 cycles), and 95% ethanol (3 cycles), respectively. The solvent was then removed under vacuum by a rotary evaporator (N-1300S, EYELA, Tokyo, Japan). Finally, twelve C. carandas extracts were obtained, i.e., n-hexane fruit extract (HF), n-hexane leaf extract (HL), n-hexane seed extract (HS), n-hexane pulp extract (HP), ethyl acetate fruit extract (AF), ethyl acetate leaf extract (AL), ethyl acetate seed extract (AS), ethyl acetate pulp extract (AP), ethanolic fruit extract (EF), ethanolic leaf extract (EL), ethanolic seed extract (ES), and ethanolic pulp extract (EP). C. carandas extracts were kept in a tight container and refrigerated (4–8 °C) until further research.
2.4. Determination of Ursolic Acid Content by High Performance Liquid Chromatography (HPLC)
Each C. carandas extract was analyzed for ursolic acid content using Hitachi HPLC series L (Tokyo, Japan) with a UV detector (Hitachi L-7420, Tokyo, Japan) set at 210 nm. A stationary phase was reverse phase column, Luna 5.0 μ C18 (2) 100A (250 × 2.0 mm, 5 μm) (Phenomenex, Torrance, CA, USA). Mixture of HPLC-grade ethanol and DI water (90:10) was used as an isocratic mobile phase with a flow rate of 0.5 mL/min. All samples were filtrated through 0.2 μm nylon filter (GNWP04700, Millipore, Darmstadt, Germany) prior to the analyzation. The injection volume of each sample was 20 μL. Ursolic acid in a concentration range of 1–500 μg/mL was used to construct a linear regression equation. The ursolic acid content of each C. carandas extracts was calculated using the following equation:
| y = 3475.3x + 5730.5 (R2 = 0.9997), | (1) |
where y was area under the curve (AUC) of ursolic acid peak which was detected around 9.3 min and x was a concentration of ursolic acid (g/mg extract). Every experiment was carried out in triplicate.
2.5. Determination of Total Phenolic Content of C. carandas Extracts by Folin–Ciocalteu Method
The total phenolic content of each C. carandas extract was evaluated using Folin–Ciocalteu method regarding to Chaiyana et al. [10], which was modified from the method of Li et al. [11]. The results were presented as gallic acid equivalent (GAE), which was milligram gallic acid equivalent per gram of each C. carandas extracts. Every experiment was carried out in triplicate.
2.6. Determination of Total Flavonoid Content of C. carandas Extracts by Dowd Method
The flavonoid content of each C. carandas extract was determined using Dowd method according to [12]. The results were reported as quercetin equivalent (QE), which was milligram quercetin equivalent per gram of each C. carandas extracts. Every experiment was carried out in triplicate.
2.7. Determination of Antioxidant Activities of C. carandas Extracts
C. carandas extracts were investigated for antioxidant activities using ferric reducing antioxidant power (FRAP) assay, 2,2′-azino-bis-3-ethylbenzthiazoline-6-sulphonic acid (ABTS) assay, and 1,1-diphenyl-2-picrylhydrazyl (DPPH) assay. Every experiment was carried out in triplicate.
2.7.1. Ferric Reducing Antioxidant Power (FRAP) Assay
The ferric reducing antioxidant power of each C. carandas extract was evaluated using the FRAP assay described by Chaiyana et al. [10], which was modified from Benzie and Strain’s method [13]. The absorbance was determined at 590 nm by a microplate reader Sp (220–1000 nm) (SPECTROstar Nano, BMG Labtech, Offenburg, Germany). Ferrous sulfate was also investigated and the resulting data were used to establish a standard curve. The results of reducing power were expressed as equivalent concentration (EC1) which represented the concentration of ferrous sulfate (mM) that exhibited an ability to reduce ferric ions (Fe3+) to ferrous ion (Fe2+) equivalent per g of C. carandas extracts.
2.7.2. 2,2′-Azino-Bis-3-Ethylbenzthiazoline-6-Sulphonic Acid (ABTS) Assay
The radical scavenging potential of each C. carandas extract was determined using an ABTS assay, as previously defined by Chaiyana et al. [10], which was adapted from Pellegrini et al. [14]. The absorbance was determined at 750 nm by a microplate reader Sp (220–1000 nm) (SPECTROstar Nano, BMG Labtech, Offenburg, Germany). Trolox was also analyzed and used for construction of a standard curve. The results were presented as a Trolox equivalent antioxidant capacity (TEAC) which represented mM concentration of Trolox solution equivalent per g of C. carandas extracts.
2.7.3. 1,1-Diphenyl-2-Picrylhydrazyl (DPPH) Assay
The radical scavenging ability of each C. carandas extract was determined using the DPPH assay Chaiyana et al. [10], which was adapted from the method defined by Blois [15]. The absorbance was determined at 520 nm by a microplate reader Sp (220–1000 nm) (SPECTROstar Nano, BMG Labtech, Offenburg, Germany). The scavenging activity of each extract was calculated from the following equation:
| DPPH inhibition (%) = {1 − [(A − B)/(C − D)]} × 100, | (2) |
where A is an UV absorbance of DPPH reagent with sample solution, B is an UV absorbance of the sample solution without DPPH reagent, C is an UV absorbance of DPPH reagent without sample solution, and D is an UV absorbance of the native solvents.
2.8. Determination of Anti-Aging Activities of C. carandas Extracts
C. carandas extracts were investigated for anti-aging activities via the determination of MMP-1, MMP-2, MMP-9, elastase, and hyaluronidase inhibition. Every experiment was carried out in triplicate.
2.8.1. Determination of MMP-1 Inhibitory Activity by Spectrophotometric Method
The MMP-1 inhibitory activity of each C. carandas extract was evaluated using spectrophotometric methods adapted from Chaiyana et al. [16] and Thring et al. [17]. The inhibitory behavior against MMP-1 was calculated from the equation:
| MMP-1 inhibition (%) = (1 − A/B) × 100, | (3) |
where A and B are the UV absorbance of the mixture with and without test sample, respectively.
2.8.2. Determination of MMP-2 and MMP-9 Inhibition by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The MMP-2 and MMP-9 inhibitory activity of each C. carandas extract was evaluated using SDS-PAGE as previously stated in the study of Chaiyana et al. [16]. Among various subclones of Albino Swiss Mouse Embryo Fibroblasts (3T3), NIH/3T3 cell line, which expressed MMP genes [18], was used in the present study. NIH-3T3 cell line was commonly used instead of skin cells because it exhibited comparable results [19]. Besides, fibroblasts are present throughout the matrix and connective tissue of the body. Therefore, NIH-3T3 cell line has been previously used for studying the regulation of fibrillar collagen synthesis, cellular cytotoxicity, genotoxicity, inhibition of UV-induced skin photoaging, inhibition of MMPs, etc., of tested compounds or topical formulations [20,21,22,23,24,25,26,27,28]. Briefly, NIH/3T3 cell line were cultured in DMEM culture medium with 10.0% newborn calf serum, and 1.0% antibiotic–antimycotic (100×). Cells were incubated in a humidified incubator with a condition of 95.0% air and 5.0% CO2 at 37.0 °C. All medium in the culture flask (2.0 mL) was removed and replaced with an equal volume of fresh medium every day. On day 7 to day 10 of culture, the medium in the culture flask was collected and then replaced with equal volume of fresh medium. The collected medium was kept in the freezer for further study.
The SDS-PAGE was then performed using a method of Chaiyana et al. [16]. MMP-2 and MMP-9 expression levels were calculated using the Quality One 1-D Analysis program (Biorad, CA, USA). The inhibitions against MMPs were calculated from the equation:
| MMPs inhibition (%) = (1 − A/B) × 100, | (4) |
where A and B are the MMPs expression of NIH/3T3 cells treated with and without sample, respectively.
2.8.3. Determination of Elastase Inhibitory Activity by Spectrophotometric Method
The inhibitory activity of each C. carandas extract against elastase was determined using a spectrophotometric approach adapted from Chaiyana et al. [16] and Thring et al. [17]. The inhibitory activity against elastase was then calculated from the equation:
| Elastase inhibition (%) = (1 − A/B) × 100, | (5) |
where A and B are the UV absorbance of the mixture with and without test sample, respectively.
2.8.4. Determination of Hyaluronidase Inhibitory Activity by Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
The SDS-PAGE was carried out in accordance with a method previously defined by Chaiyana et al. [16]. Briefly, hyaluronidase was incubated with or without the extract at 37 °C for 48 h. The resulting mixture was then mixed with loading dye and loaded into each well of SDS-polyacrylamide gel containing 0.17% hyaluronic acid. After applying the voltage, the bands gently moved down and hyaluronidase was separated. Along with the enzyme separation, the digested hyaluronic acid by hyaluronidase was removed from the gel. Then the gel was washed, incubated in reaction buffer pH 5.0 for 16 h, soaked in staining buffer of Alcian blue for 1 h, and incubated in de-staining buffer until the protein marker showed up. Since hyaluronidase digested hyaluronic acid in the gel, the results would be detected as a clear area. In contrast, if a sample exhibited anti-hyaluronidase activity, its substrate would not be digested, and no clear area detected. The hyaluronidase expression was determined using the Quality One 1-D Analysis program (Biorad, CA, USA). The inhibitions against hyaluronidase were calculated from the equation:
| Hyaluronidase inhibition (%) = (1 − A/B) × 100, | (6) |
where A and B are the hyaluronic acid expression after treated with sample and without sample, respectively.
2.9. Anti-Inflammatory Activity Determination
C. carandas extracts were investigated for anti-inflammatory activities via the determination of NF-κB, IL-6, and TNF-α inhibition. Every experiment was carried out in triplicate.
2.9.1. Determination of NF-κB Expression by Western Blot Analysis
Firstly, the cell viability of U937 cells (American Type Culture Collection, VA, USA) was investigated using the MTT colorimetric method, which was slightly modified from the previous analysis of Anuchapreeda et al. [29]. U937 cell viability was calculated from the equation:
| Cell viability (%) = (1 − A/B) × 100, | (7) |
where A and B are the optical density of vehicle control and test sample, respectively. The concentration of each C. carandas extract showing 80% of cell viability was selected for the further immunoblotting study. Every experiment was carried out in triplicate. The level of NF-κB expression was measured using Western blotting, as defined previously by Chaiyana et al. [30]. Indomethacin, generally known as nonsteroidal anti-inflammatory drug (NSAID), was used as a positive control.
2.9.2. Determination of IL-6 and TNF-α Secretion by Enzyme-Linked Immunosorbent Assay (ELISA)
IL-6 and TNF-α secretion was determined by ELISA according to the method of Chaiyana et al. [16]. Dexamethasone, generally known as a steroid used for anti-inflammation, was used as a positive control. On the other hand, negative control was the LPS-induced RAW 264.7 cells (American Type Culture Collection, VA, USA) treated without sample and vehicle control was the RAW 264.7 cells treated with nothing. The cell viability of RAW 264.7 cells was measured using the MTT colorimetric technique, which was modified from the methods previously defined by Chaiyana et al. [16] and Mueller et al. [31]. RAW 264.7 cells viability was then calculated from the equation:
| Cell viability (%) = (1 − A/B) × 100, | (8) |
where A is an optical density of vehicle control and B is an optical density of sample, positive control, or negative control. To limit variability due to cell density differences, IL-6 and TNF-secretions were standardized to MTT values [31]. The secretions of those cytokines from negative control, which were RAW 264.7 cells treated with only LPS, was described as 100%. Additionally, % inhibition against IL-6 and TNF-α was calculated by subtracting the cytokines secretion from 100%.
2.10. Anti-Tyrosinase Determination by Dopachrome Method
The anti-tyrosinase activity of each C. carandas extract was determined using DOPAchrome method regarding to a method of Momtaz et al. [32] which was adapted from Curto et al. [33] and Nerya et al. [34]. As substrates, L-tyrosine and L-DOPA were used. As a positive control, kojic acid was used. The inhibitory activity against tyrosinase was calculated from the equation:
| Tyrosinase inhibition (%) = (1 − A/B) × 100, | (9) |
where A and B are the absorbance of the mixture with and without test sample, respectively. Every experiment was carried out in triplicate.
2.11. Cytotoxicity of C. carandas Extracts in Human Keratinocyte (Hacat) Cells by MTT Colorimetric Technique
The cell viability of sample-treated HaCaT cells (American Type Culture Collection, VA, USA) was calculated using the MTT colorimetric procedure, which was modified from the methods of Colombo et al. [35] and Anuchapreeda et al. [29]. The viability of HaCaT cells was investigated using the MTT colorimetric method. HaCaT cell viability was calculated from the equation:
| Cell viability (%) = (1 − A/B) × 100, | (10) |
where A and B are the optical density of vehicle control and test sample, respectively. The concentration of each C. carandas extract showing 80% of cell viability was selected for the further immunoblotting study. Every experiment was carried out in triplicate.
2.12. Statistical Analysis
All data were provided in the form of a mean ± standard deviation (S.D.). Individual differences were assessed using one-way analysis of variance (ANOVA), which was supplemented by Tukey’s post-hoc analysis. * p < 0.05, ** p < 0.01, and *** p < 0.001 indicated statistical significance.
3. Results and Discussion
3.1. C. carandas Extracts
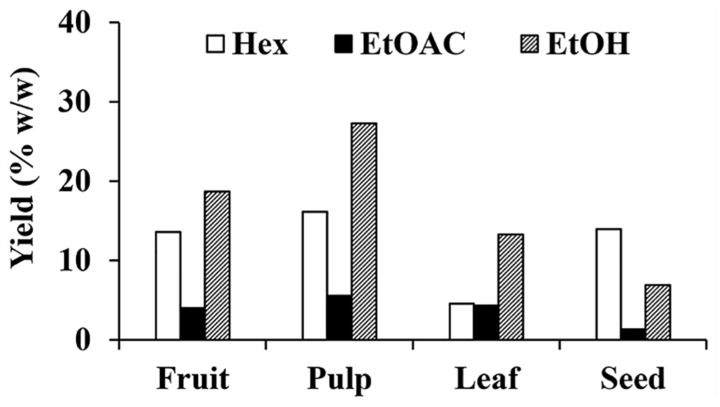
The yields of each C. carandas extracts are shown in Figure 1. n-Hexane and ethanolic extracts were a semisolid mass. On the other hand, all ethyl acetate extracts were solid powder. The color of each extract depended on the C. carandas materials, e.g., the extracts from the leaf, seed, pulp, and fruit part had a dark green, yellow–brown, dark purple, and dark brown color, respectively. Interestingly, ethanolic extracts showed the significantly highest yields in fruit, pulp, and leaf part of C. carandas, whereas the significantly highest yield of the extracts from seed part was obtained from the n-hexane extracts. The likely explanation was due to the high content of fatty acid, i.e., palmitic acid and stearic acid, in the seed part [4] but high content of phenolic compounds in the others [36,37]. Therefore, it was likely that C. carandas fruit, pulp, and leaf contained polar phytoconstituents which were extracted well by polar solvent. In contrast, the C. carandas seed contained nonpolar phytoconstituents which was extracted well by nonpolar solvent.
Figure 1.
Yields of C. carandas extracts sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively.
Among twelve C. carandas extracts, PE showed the highest yield which was 27.3% w/w. Besides, the pulp part yielded the highest extract amount in all extracted solvent used. The likely explanation was because an edible pulp is rich with several bioactive compounds and mineral contents.
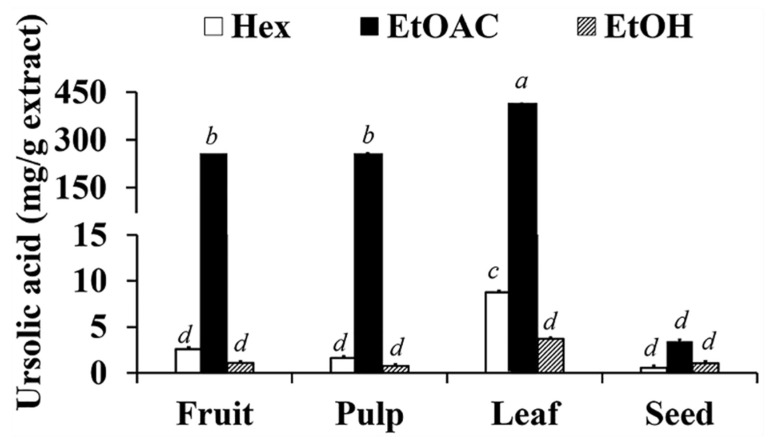
3.2. Ursolic Acid Content of C. carandas Extracts
The ursolic acid content of C. carandas extracts are shown in Figure 2. The results indicated that ethyl acetate was the most suitable solvent for ursolic acid extraction since the ursolic acid contents were significantly highest in all ethyl acetate extracts, including the extract from fruit, leaf, pulp, and seed part (p < 0.05). Although the previous ursolic acid solubility study noted that ursolic acid was well dissolved in ethanol (solubility = 16.808 ± 0.824 mg/mL), followed by ethyl acetate (solubility = 6.857 ± 0.359 mg/mL) and n-hexane (solubility = 0.471 ± 0.064 mg/mL) [38,39], in this study it was revealed that ethyl acetate extracts contained a significantly higher amount of ursolic acid compared to ethanolic extracts. The reasons were due to a subsequential extraction process. Since the C. carandas dried powder was macerated in ethyl acetate before ethanol, ursolic acid was therefore extracted and existed in the ethyl acetate extract, leading to lower ursolic acid content detected in ethanolic extracts.
Figure 2.
Ursolic acid content of C. carandas extracts sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively. The letter a, b, c, and d denote significant difference among different C. carandas extracts (p < 0.05) after being analyzed by one-way ANOVA followed by Tukey’s test.
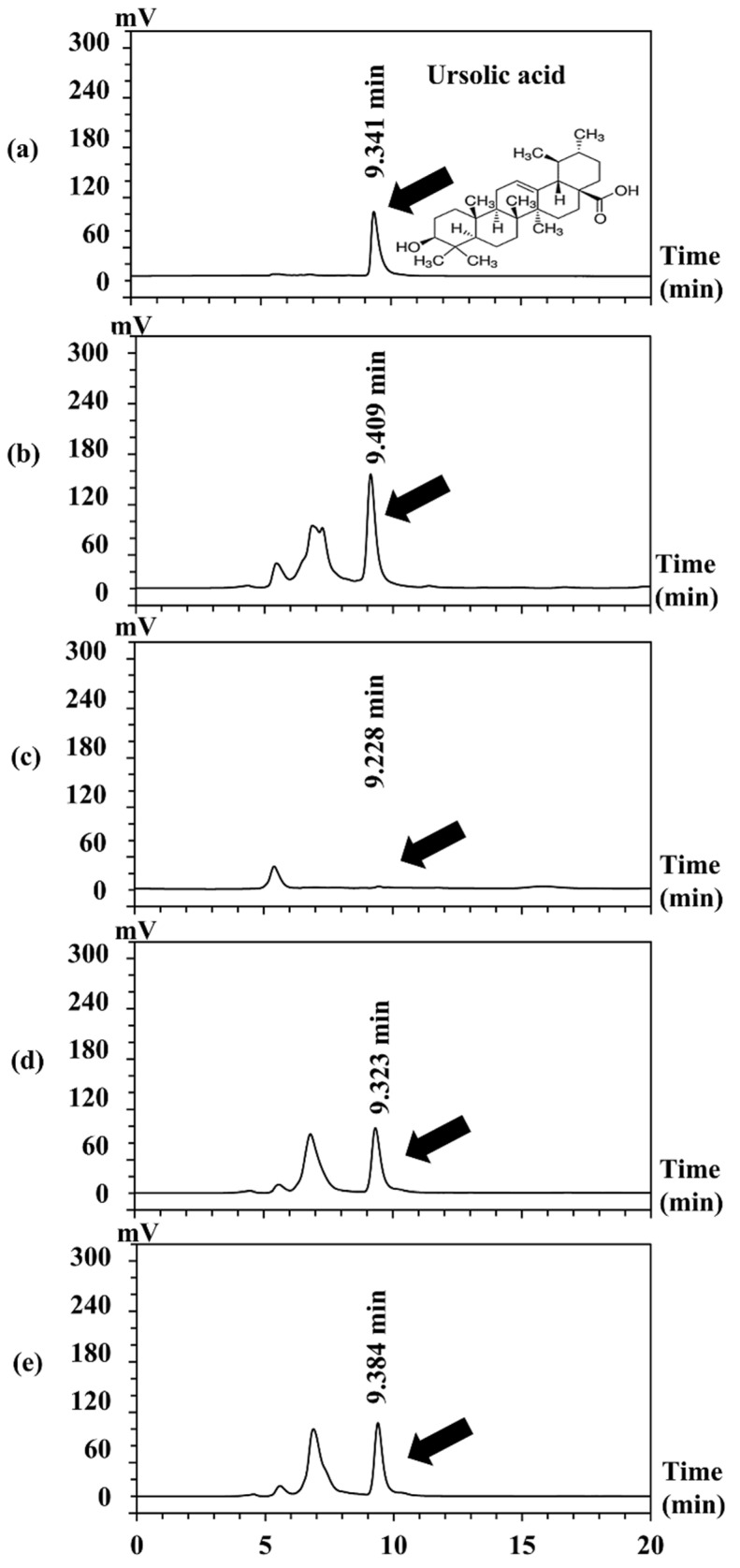
Among various parts of C. carandas extracted using ethyl acetate, AL contained the significantly highest ursolic acid (411.8 mg/g extract), followed by AP (256.34 mg/g extract), AF (253.32 mg/g extract), and AS (3.4 mg/g extract), respectively (p < 0.05). The HPLC chromatograms of ethyl acetate extracts from different part of C. carandas are shown in Figure 3. The major peaks detected in chromatograms of C. carandas ethyl acetate extracts were at the retention time ranging from 9.228 to 9.409 min which were close to that of ursolic acid (9.341 min). Therefore, it could be concluded that ursolic acid was a main abundant compound in C. carandas extracts.
Figure 3.
HPLC chromatograms of ursolic acid (a) and ethyl acetate extracts from various parts of C. carandas, i.e., leaf (b), seed (c), pulp (d), and fruit (e).
On the other hand, among various part of C. carandas extracted using n-hexane, HL contained the significantly highest ursolic acid (8.77 mg/g extract) (p < 0.05), whereas HF (2.54 mg/g extract), HP (1.60 mg/g extract), and HS (0.55 mg/g extract) contained no significantly different ursolic acid content (p > 0.05). In contrast, different part of C. carandas extracted using ethanol yielded no significant difference in ursolic acid content (p > 0.05).
Since ursolic acid has been reported for several pharmacological activities, such as anti-inflammation, anti-tumor, and antibacterial activities [40], it could be used as a marker for further quantitative analysis of C. carandas extract.
3.3. Total Phenolic and Flavonoid Content of C. carandas Extracts
TPC and TFC of C. carandas extracts are shown in Table 1. Among various solvents, ethanolic extracts yielded the significantly highest phenolic and flavonoid content comparing to the others (p < 0.05). Therefore, ethanol was noted as the most suitable solvent for extracting phenolics and flavonoids. The likely explanation was due to its hydrophilicity. The previous study reported that the highest TPC was obtained from extraction using methanol (ε = 33.0), followed by ethanol (ε = 25.3) and acetone (ε = 20.7), respectively [41]. Although more polar solvent could extract more phenolic compounds, ethanol was used in the present study and suggested for further study since it is widely known as safe and low cost. Additionally, ethanol has been reported to extract more flavonoids comparing to methanol and acetone [41].
Table 1.
Total phenolics content, total flavonoids content, and antioxidant activities of each C. carandas extracts.
| Samples | GAE (mg Gallic Acid/g Sample) | QE (mg Guercetin/g Sample) | Antioxidant Activities | |||
|---|---|---|---|---|---|---|
| EC1 (µM FeSO4/g Extract) | TEAC (µM Trolox/g Extract) | IC50 on DPPH•+ (µg/mL) | ||||
| Ursolic acid | ND | ND | 0.0 ± 0.0 f | 5.1 ± 2.1 e | >125.0 e | |
| Gallic acid | ND | ND | 22,873 ± 203 a | 25.0 ± 2.6 c | 1.0 ± 0.0 d | |
| Quercetin | ND | ND | 81,461 ± 308 b | 58.1 ± 2.7 a | 4.4 ± 0.4 d | |
| Hex | 0.5 ± 4.4 e | 1.7 ± 0.1 e | 0.0 ± 11.6 f | 15.1 ± 2.1 e | >125.0 e | |
| Fruits | EtOAc | 19.8 ± 3.6 d | 4.1 ± 0.5 d | 959.5 ± 10.8 f | 85.5 ± 7.4 d | 420.8 ± 57.4 a |
| EtOH | 61.2 ± 4.4 c | 10.1 ± 0.3 b | 470.0 ± 48.7 e | 251.7 ± 13.1 c | 414.5 ± 20.0 a,b | |
| Hex | 0.2 ± 3.6 e | 1.2 ± 0.0 e | 0.0 ± 5.5 f | 17.2 ± 8.8 e | >125.0 e | |
| Pulp | EtOAc | 22.6 ± 2.3 d | 2.5 ± 0.1 e | 140.9 ± 10.5 f | 103.6 ± 9.8 d | 312.3 ± 45.7 a–c |
| EtOH | 24.2 ± 1.0 d | 14.1 ± 0.2 a | 198.5 ± 5.6 e,f | 131.8 ± 3.9 d | 224.7 ± 27.8 b,c | |
| Hex | 6.0 ± 2.2 e | 4.2 ± 0.2 d | 0.0 ± 5.6 f | 42.2 ± 27.1 e | >125.0 e | |
| Leaf | EtOAc | 18.3 ± 2.0 d | 4.8 ± 0.2 d | 165.6 ± 14.2 f | 123.5 ± 6.5 d | 301.8 ± 45.6 b |
| EtOH | 219.7 ± 4.8 b | 8.8 ± 2.2 b | 3,531 ± 159 c | 496.0 ± 6.2 b | 16.4 ± 0.1 d | |
| Hex | 0.0 ± 0.7 e | 1.0 ± 0.1 e | 35.0 ± 1.9 f | 22.2 ± 4.8 e | >125.0 e | |
| Seed | EtOAc | 15.4 ± 1.3 d | 7.5 ± 0.2 b | 86.1 ± 10.8 e,f | 138.7 ± 10.1 d | >125.0 e |
| EtOH | 265.1 ± 1.4 a | 6.8 ± 0.1 c | 2,228 ± 337 d | 492.8 ± 4.1 b | 16.0 ± 0.5 d | |
NOTE: HEX = n-hexane extracts; EtOAc = ethyl acetate extracts; EtOH = ethanolic extract. The letters a–f denote significant difference among different C. carandas extracts (p < 0.05) after being analyzed by one-way analysis of variance (ANOVA) followed by Tukey’s test. ND refers to not determined.
Among ethanolic extracts from different C. carandas parts, ES yielded the significantly highest phenolic content with the GAE of 265.1 ± 1.4 mg/g extract, followed by EL (219.7 ± 4.8 mg/g extract), EF (61.2 ± 4.4 mg/g extract), and EP (24.2 ± 1.0 mg/g extract), respectively. Interestingly, the present study is the first to report a remarkable phenolic content in C. carandas seed.
On the other hand, EP yielded the significantly highest flavonoid content with the QE of 14.1 ± 0.2 mg/g extract, followed by EF (10.1 ± 0.3 mg/g extract), EL (8.8 ± 2.2 mg/g extract), and ES (6.8 ± 0.1 mg/g extract), respectively. The results noted that other than ursolic acid, triterpenoid, phenolics, and flavonoids were also detected in various parts of C. carandas. The results were well accordant with previous studies which reported that various phenolics and flavonoids have been detected in C. carandas, especially vanillic acid in fruit, as well as ellagic acid and quercetin in pulp [37,42,43].
3.4. Antioxidant Activities of C. carandas Extracts
Ethanolic extract from various part of C. carandas possessed the highest antioxidants activity via both scavenging and reducing abilities as shown in Table 1. Among different ethanolic extracts, EL possessed the significantly highest antioxidant activities with EC1 of 3.53 ± 0.16 mM/g extract, TEAC value of 496.0 ± 6.2 µM/g extract, and IC50 against DPPH• of 16.2 ± 0.1 µg/mL (p < 0.05). Additionally, ES also showed comparable ABTS•+ and DPPH• scavenging activity to EL with TEAC value of 492.8 ± 4.1 µM/g extract and IC50 against DPPH• of 16.0 ± 0.5 µg/mL (p > 0.05). The antioxidant results were well accordant with TPC, but not related to TFC and ursolic acid content. Therefore, it could be concluded that phenolics were major compounds responsible for the antioxidant properties of C. carandas extracts. Since research has proposed that topical antioxidants be used for the treatment of skin aging [44,45,46], C. carandas extracts, especially EL, have been suggested to be used as natural antioxidants, which could prevent skin aging. A previous study reported that antioxidants play an important role in the prevention and treatment of UV-induced skin aging due to the reduction in oxidized proteins, which strongly correlates with the severity of photo-aging clinical characteristics [47].
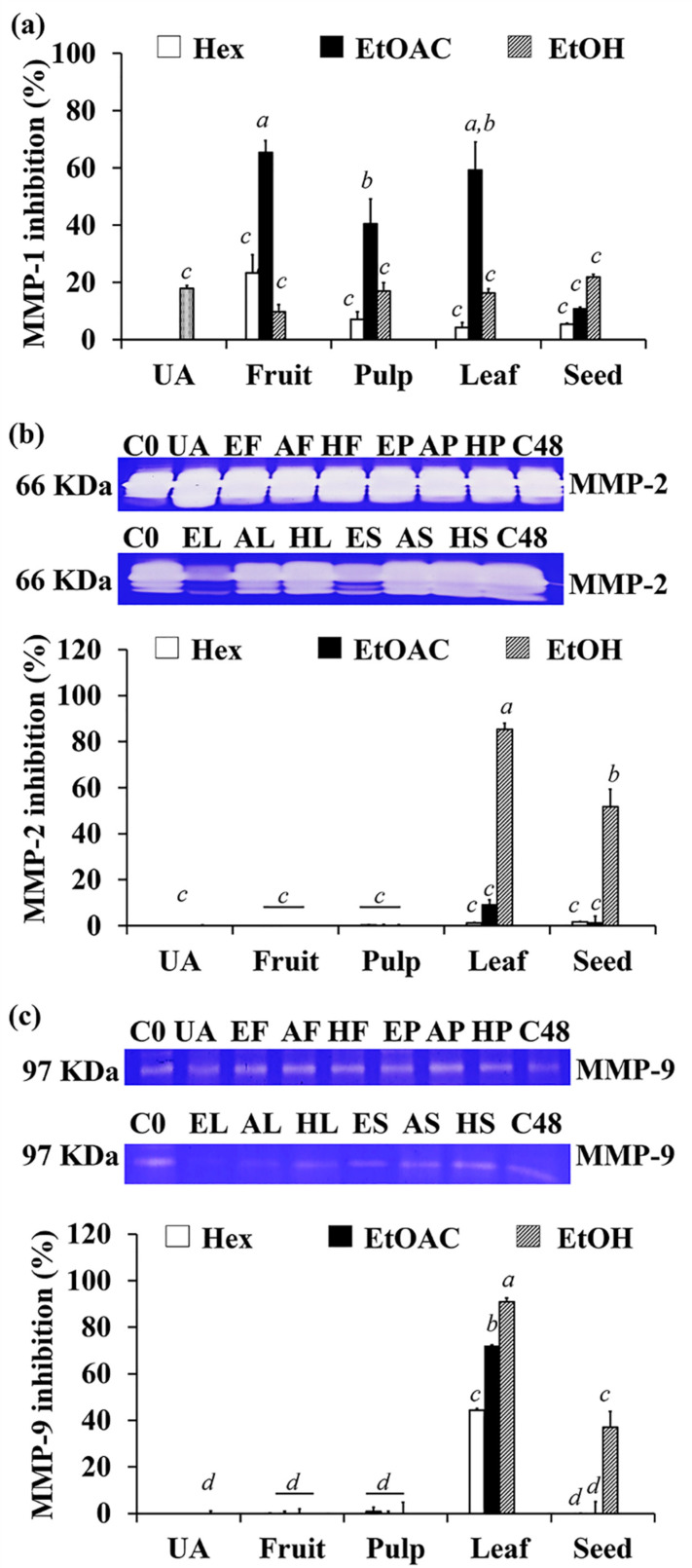
3.5. MPPs Inhibitory Activities of C. carandas Extracts
MMPs are a form of zinc-dependent endopeptidase that degrades ECM components and is involved in dermal turnover and remodeling [48]. There are several different types of MMPs, but only a few have been associated to skin wrinkles, including collagenases, which break down collagens (MMP-1), and gelatinases, which degrade denatured collagens (MMP-2 and MMP-9) [49]. The inhibitory activities of C. carandas extracts against these MMPs are shown in Figure 4. There were multiple bands detected around 66 KDa because MMP-2 could be present in both pro MMP-2 and the active form. Pro MMP-2 in its full-length of 72 kDa could be activated by proteolytic cleavage to an enzymatically active form, which was around 62–67 kDa [50,51,52]. Since the size of pro MMP-2 and its active form were very close, the resultant band was oversaturated and overlapped. However, the results were calculated from the summation of all bands detected around 66 KDa.
Figure 4.
Inhibition against MMP-1 (a), MMP-2 (b), and MMP-9 (c) of 1 mg/mL ursolic acid (UA) and various parts of C. carandas sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively. DMSO was used as a vehicle control at 0 h (C0) and 48 h (C48). Various C. carandas extracts included n-hexane extracts from fruit (HF), leaf (HL), seed (HS), and pulp (HP); ethyl acetate extracts from fruit (AF), leaf (AL), seed (AS), and pulp (AP); ethanolic extracts from fruit (EF), leaf (EL), seed (ES), and pulp (EP). The letter a, b, c, and d denote significant difference among different C. carandas extracts (p < 0.05) after analyzed by one-way ANOVA followed by Tukey’s test. ND refer to not detected value.
Ethyl acetate extracts tended to possess significantly higher MMP-1 inhibition, whereas ethanolic extracts tended to possess significantly higher MMP-2 and MMP-9 inhibition (p < 0.05). Among ethyl acetate extracts, AF and AL possessed the significantly highest inhibition against MMP-1 (p < 0.05) with the inhibition of 65.4 ± 4.0% and 59.2 ± 9.8%, respectively (p < 0.05). On the other hand, EL possessed the significantly highest inhibition against both MMP-2 and MMP-9 with the inhibition of 85.3 ± 2.8% and 90.2 ± 1.6%, respectively (p < 0.05). Although the gelatin zymography results of MMP-2 are oversaturated, making it difficult to confirm the exact results, the inhibitions against MMP-2 were distinctly observed in EL and ES. The likely explanation lies in the different hemopexin-like C-terminal domain in MMP-1, MMP-2, and MMP-9 that conducted the specific binding site for substrates and inhibitors since MMPs are composed of several active domains, including the prodomain, catalytic domain, hemopexin domain, and hinge region [16,53,54]. The specific site three-quarters from the N-terminal of the hemopexin domain in MMP-1 plays an important role in cleavage of substrates such as collagens type I, II, and III [53,54], while MMP-2 and MMP-9 have three repeats of a type II fibronectin domain embedded in the catalytic domain, and the groove in the hemopexin domain helps with substrate binding [16,53]. Therefore, it could be summarized that the ethyl acetate extracts (especially AF and AL) were more specific for MMP-1 inhibition, whereas the ethanolic extract (especially EL) were more specific for MMP-2 and MMP-9 inhibition. However, these C. carandas extracts were suggested for the prevention of skin aging caused by UV-A irradiation. Since UV-A irradiation up-regulated the levels of MMP-1, MMP-2, and MMP-9, which are crucial in the breakdown of almost all extracellular matrix components and result in the decomposition of dermal structures and finally skin aging [55].
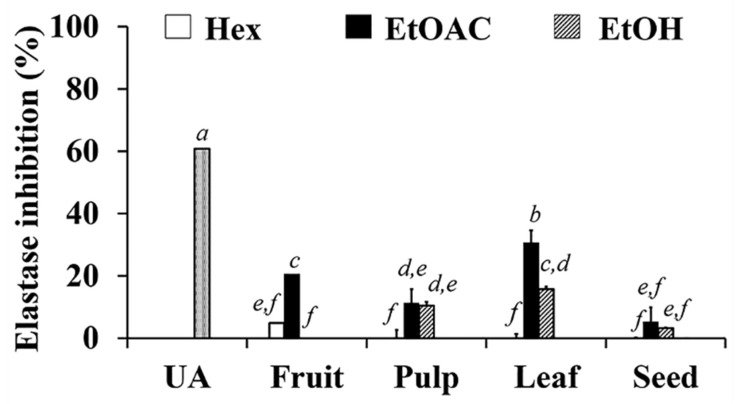
3.6. Elastase Inhibitory Activities of C. carandas Extracts
Elastase degrades elastic fibers, which are mostly found in connective tissue and make up the extracellular matrix that gives skin its elasticity; thus, elastase degeneration is linked to the formation of wrinkles [56,57]. The elastase inhibition of C. carandas extracts are shown in Figure 5. Ethyl acetate extracts, particularly the ethyl acetate extract from leaves, were found to be the most effective elastase inhibitors (p < 0.05). Ursolic acid was identified as a compound responsible for the elastase inhibitory activity since it possessed a strong inhibition of 60.8 ± 4.0%. Furthermore, the elastase inhibition was greatest in EL, which had the highest ursolic acid content. The findings were consistent with prior research that found ursolic acid inhibited human and porcine leucocyte elastase by binding to the enzyme’s subsites S3–S5 [58,59]. As a result, EL could be a promising active compound for preventing elastase degradation and improving skin elasticity.
Figure 5.
Inhibition against elastase of 1 mg/mL ursolic acid (UA) and various parts of C. carandas sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively. DMSO was used as a vehicle control. The letter a, b, c, d, e, and f denote significant difference among different C. carandas extracts (p < 0.05) after analyzed by one-way ANOVA followed by Tukey’s test.
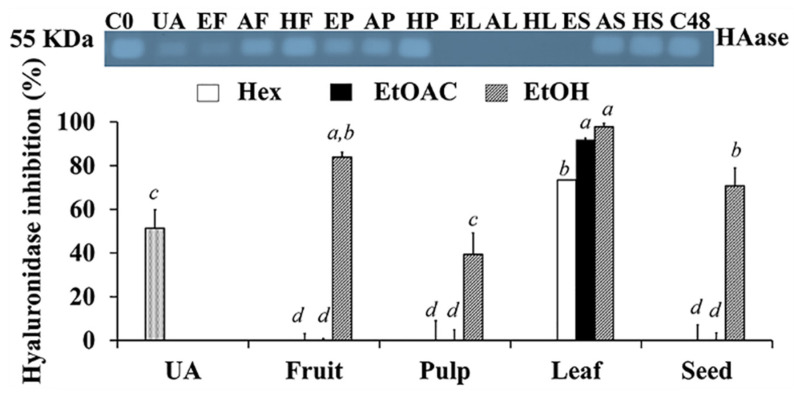
3.7. Hyaluronidase Inhibitory Activity of C. carandas Extracts
Hyaluronidase is a key hydrolysis enzyme that cleaves the 1,4-glycosidic bond in hyaluronan, of which 50% is found in skin tissue [60]. Because of its high hydration capacity, hyaluronan, which is produced by epidermal keratinocytes and dermal fibroblasts, contributes to the skin’s viscoelastic properties [61,62]. Therefore, hyaluronan degradation in the skin by hyaluronidase resulted in a loss of skin moisture and laxity, which are all signs of aging skin. Compounds that inhibited hyaluronan turnover by inhibiting hyaluronidase activity were helpful for skin rejuvenation.
The inhibitory activity against hyaluronidase of C. carandas extracts are shown in Figure 6. The ethanolic C. carandas extracts exhibited outstanding hyaluronidase inhibition, particularly from leaves (97.8 ± 1.6%), fruit (83.9 ± 2.2%), and seed (70.7 ± 8.2%), all of which were higher than ursolic acid (51.3 ± 8.5%). The findings were consistent with our previous research, which found that ursolic acid could inhibit hyaluronidase with a 40–60% inhibition at a concentration of 1 mg/mL [16]. Therefore, ursolic acid might not be the only compound in C. carandas that is responsible for the hyaluronidase inhibition. Other phenolics and flavonoids contained in C. carandas may be compounds that inhibit hyaluronidase, as the ethanolic extracts with high levels of phenolics and flavonoids significantly inhibited the enzyme hyaluronidase. The previous structure–activity studies reported that flavonoids, such as luteolin, apigenin, and kaempferol were found to have high inhibitory activity against hyaluronidase, while silybin, myricetin, and quercetin were found to have moderate inhibitory activity [63,64]. Therefore, phenolic and flavonoids from C. carandas extracts would be the compounds for inhibiting hyaluronidase.
Figure 6.
Inhibition against hyaluronidase of 1 mg/mL ursolic acid (UA) and various parts of C. carandas sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively. DMSO was used as a vehicle control at 0 h (C0) and 48h (C48). Various C. carandas extracts included n-hexane extracts from fruit (HF), leaf (HL), seed (HS), and pulp (HP); ethyl acetate extracts from fruit (AF), leaf (AL), seed (AS), and pulp (AP); ethanolic extracts from fruit (EF), leaf (EL), seed (ES), and pulp (EP). The letters a, b, c, and d denote significant difference among different C. carandas extracts (p < 0.05) after being analyzed by one-way ANOVA followed by Tukey’s test.
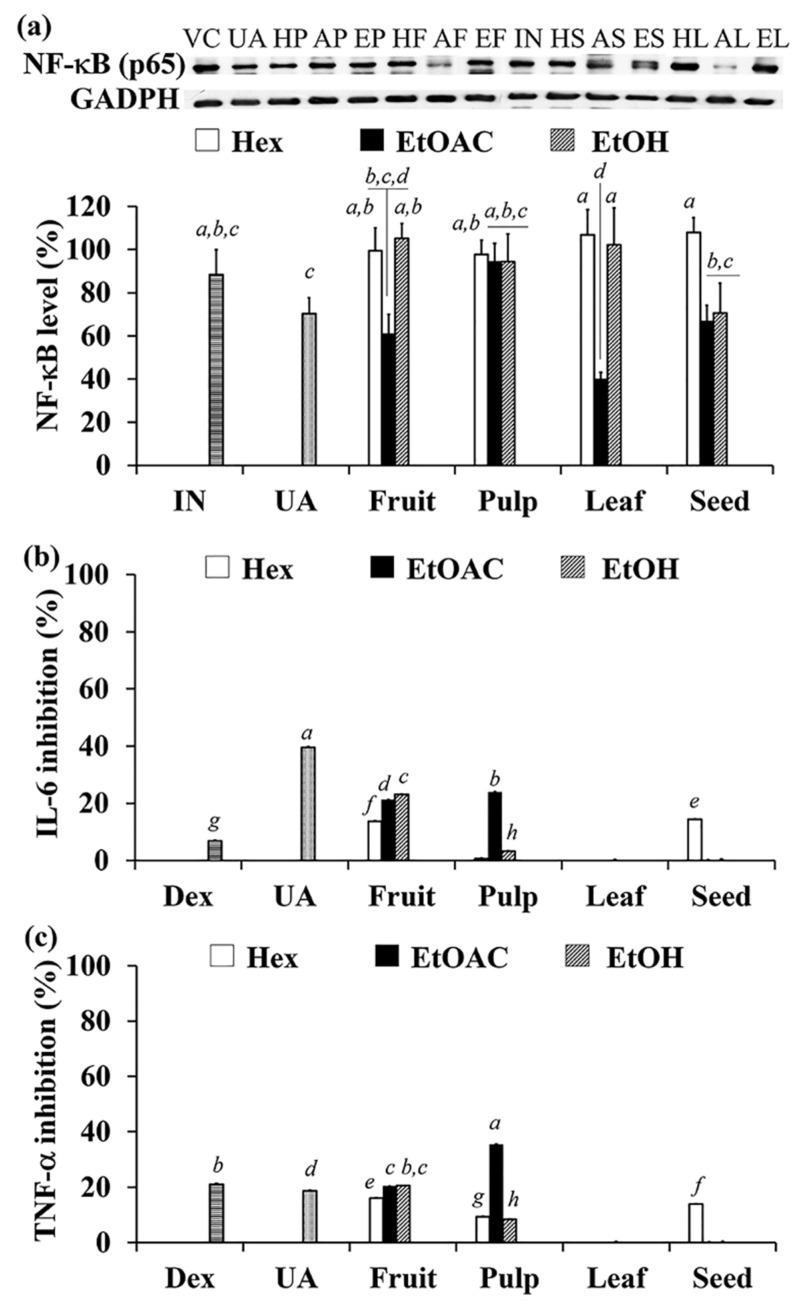
3.8. Anti-Inflammatory Activity of C. carandas Extracts
Inflammation underlies a broad range of pathological and physiological processes [65]. NF-κB is released and translocated to the nucleus after the phosphorylation of the inhibitory IκB, and consequently regulates the expression of many genes involved in inflammatory signaling, cell proliferation, and cell apoptosis. Because the nuclear factor NF-κB signaling pathway has long been considered to be a model of proinflammatory signaling, NF-κB inhibitors may be useful as anti-inflammatory agents [66]. In addition, the inflammatory cytokines (TNF-α, IL-6, and others), which are primarily produced by macrophages and mast cells, play a number of functions in the inflammatory response, including stimulating endothelial cells and leukocytes, as well as triggering the acute-phase response [65].
The anti-inflammatory activities of C. carandas extracts indicated that several C. carandas extracts displayed potent anti-inflammatory activities, as shown in Figure 7. The IC20 values (Table 2) represented the concentrations at 80% of viable U937 or RAW 264.7 cells. Each C. carandas extract at IC20 value was used in the assessment of anti-inflammatory activity. n-Hexane extracts were the safest extracts since they exhibited the highest value of IC20 which were greater than 250 µg/mL in U937 cells and 100 µg/mL in RAW 264.7 cells. Except for the leaf part, ethyl acetate extracts had greater effects on U937 and RAW 264.7 cells viabilities than ethanolic extracts.
Figure 7.
The effect of ursolic acid (UA), and C. carandas sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively, on NF-κB protein expressions in U937 cells (a), IL-6 (b) and TNF-α (c) secretion in RAW 264.7 cells. Various C. carandas extracts included n-hexane extracts from fruit (HF), leaf (HL), seed (HS), and pulp (HP); ethyl acetate extracts from fruit (AF), leaf (AL), seed (AS), and pulp (AP); ethanolic extracts from fruit (EF), leaf (EL), seed (ES), and pulp (EP). Dimethyl sulfoxide was used as vehicle control (VC). Indomethacin (IN) and dexamethasone (Dex) were used as reference standard. The letters a, b, c, d, e, f, g, and h denote significant difference among different C. carandas extracts (p < 0.05) after being analyzed by one-way ANOVA followed by Tukey’s test.
Table 2.
IC20 of each C. carandas extracts on U937 cells and RAW 264.7 cells.
| C. carandas Extracts | IC20 (µg/mL) | ||
|---|---|---|---|
| U937 Cells | RAW 264.7 Cells | ||
| Ursolic acid | 9.6 ± 1.1 | 8.2 ± 0.3 | |
| Hex | >250 | >100 | |
| Fruits | EtOAc | 31.9 ± 6.7 | 15.9 ± 3.7 |
| EtOH | 40.8 ± 3.5 | >100 | |
| Hex | >250 | >100 | |
| Pulp | EtOAc | 36.1 ± 8.0 | 20.9 ± 0.5 |
| EtOH | 118.0 ± 12.6 | >100 | |
| Hex | >250 | >100 | |
| Leaf | EtOAc | 52.2 ± 3.7 | 15.0 ± 0.5 |
| EtOH | 49.6 ± 3.5 | >100 | |
| Hex | >250 | >100 | |
| Seed | EtOAc | 7.4 ± 2.0 | >100 |
| EtOH | 34.6 ± 8.8 | >100 | |
Note: HEX = n-hexane extracts; EtOAc = ethyl acetate extracts; EtOH = ethanolic extract.
AL was significantly the most effective inhibiting NF-κB expression with the inhibition of 59.9 ± 3%, which was more potent than ursolic acid (29.7 ± 7.3% inhibition) and indomethacin, a well-known nonsteroidal anti-inflammatory drug (11.6 ± 11.6% inhibition) (p < 0.05). This suggests that C. carandas extracts contain other compounds that suppress NF-κB protein expression in addition to ursolic acid. In contrast, AP showed the highest inhibitory effects on IL-6 and TNF-α secretions with inhibitory values of 23.9 ± 0.2% and 35.4 ± 0.2%, respectively, which were significantly more potent than dexamethasone (p < 0.05) at the same concentration (10 µg/mL). Ursolic acid, a major component of C. carandas, was shown to be responsible for IL-6 and TNF-α inhibition, as it had a stronger inhibitory effect than dexamethasone.
The finding from this study remarked that the extracts with the most potent inhibitory activity on NF-κB had no effect on IL-6 or TNF-α secretion inhibition, while the extract with the most potent inhibitory activity on IL-6 and TNF-α secretion had no effect on NF-κB suppression. Although the NF-κB pathway is one of the intracellular signaling pathways to activate IL-6 and TNF-α, there are varieties of mechanisms that would also be involved, such as mammalian mitogen-activated protein kinase (MAPK), Janus kinase 2/signal transducers and activators of transcription 3(JAK2/STAT3), etc. [67,68,69]. Inflammation can be resolved through many mechanisms. In this study, NF-κB and IL-6 were examined in different cell lines (U937 and Raw 264.7 cells, respectively). Thus, it is possible that the result did not relate to each other. NF-IL6 (C/EBP) and NF-κB are proteins involved in IL-6 gene expression in U937 cells [70]. However, Raw 264.7 showed a different signaling pathway that associates with NF-κB and ERK (MAPK) [71,72].
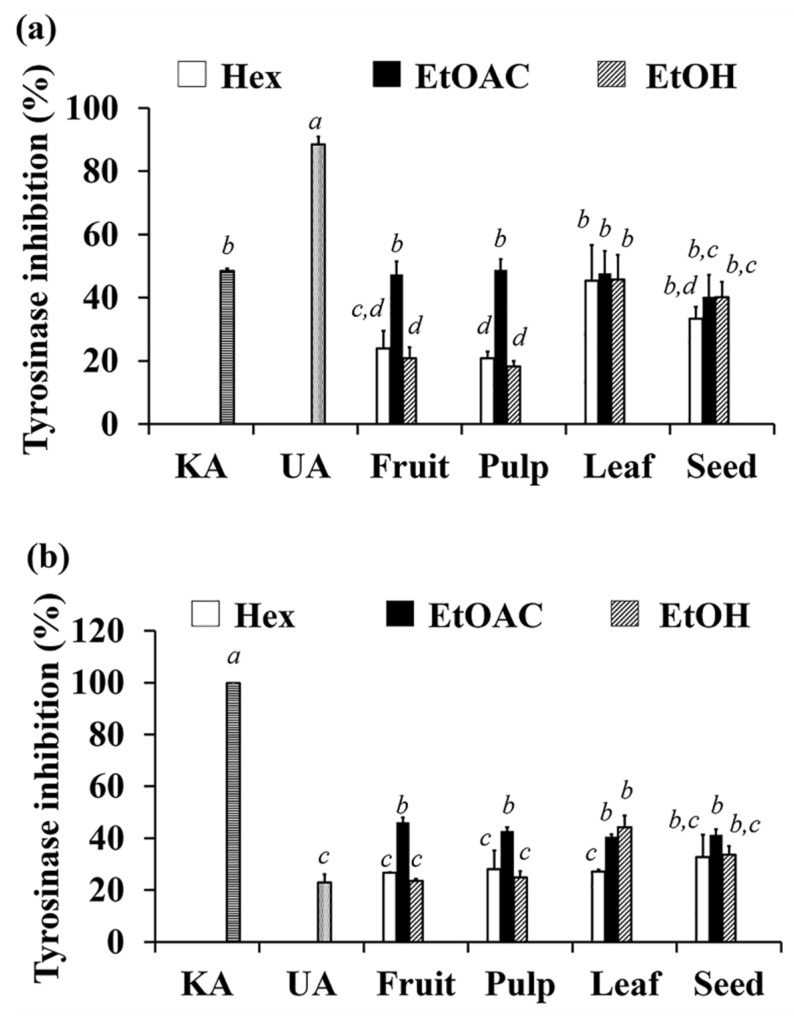
3.9. Anti-Tyrosinase Activity of C. carandas Extracts
The anti-tyrosinase activities of C. carandas extracts are shown in Figure 8. The diphenolase (Figure 8a) and monophenolase (Figure 8b) activities of mushroom tyrosinase were evaluated using L-DOPA and L-tyrosine as substrates, respectively.
Figure 8.
Inhibition against diphenolase (a) and monophenolase (b) activities of tyrosinase at concentration of 125 µg/mL kojic acid (KA), ursolic acid (UA) and C. carandas extracts sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively. The letters a, b and c denote significant difference among different C. carandas extracts (p < 0.05) after being analyzed by one-way ANOVA followed by Tukey’s test.
The significantly greatest inhibitory activity against tyrosinase was found in ethyl acetate extracts from different parts of C. carandas (p < 0.05). AF (47.3 ± 4.2%), AP (48.9 ± 3.3%), AL (47.7 ± 7.1%), and AS (40.3 ± 6.9%) were effective diphenolase inhibitors with anti-tyrosinase activity not significantly different from that of kojic acid (48.4 ± 0.4%). Ursolic acid was a key component in their anti-tyrosinase efforts, as it exhibited the highest inhibition of 88.5 ± 2.4% (p < 0.05).
Similarly, AF (46.2 ± 1.8%), AP (42.9 ± 1.2%), AL (40.5 ± 1.0%), and AS (41.2 ± 2.0%), were the most effective on monophenolase inhibition. But their monophenolase inhibition were higher than that of ursolic acid (22.9 ± 3.1%). It would be explainable that other compounds than ursolic acid may have been responsible for the monophenolase inhibition. Although ursolic acid was more selective to diphenolase than monophenolase, all ethyl acetate extracts were effective on both diphenolase and monophenolase. Therefore, another benefit of natural extract was the synergistic effects of many components contained in the extract, which made them more potent at the same concentration as the pure compound.
3.10. HaCaT Cell Cytotoxicity
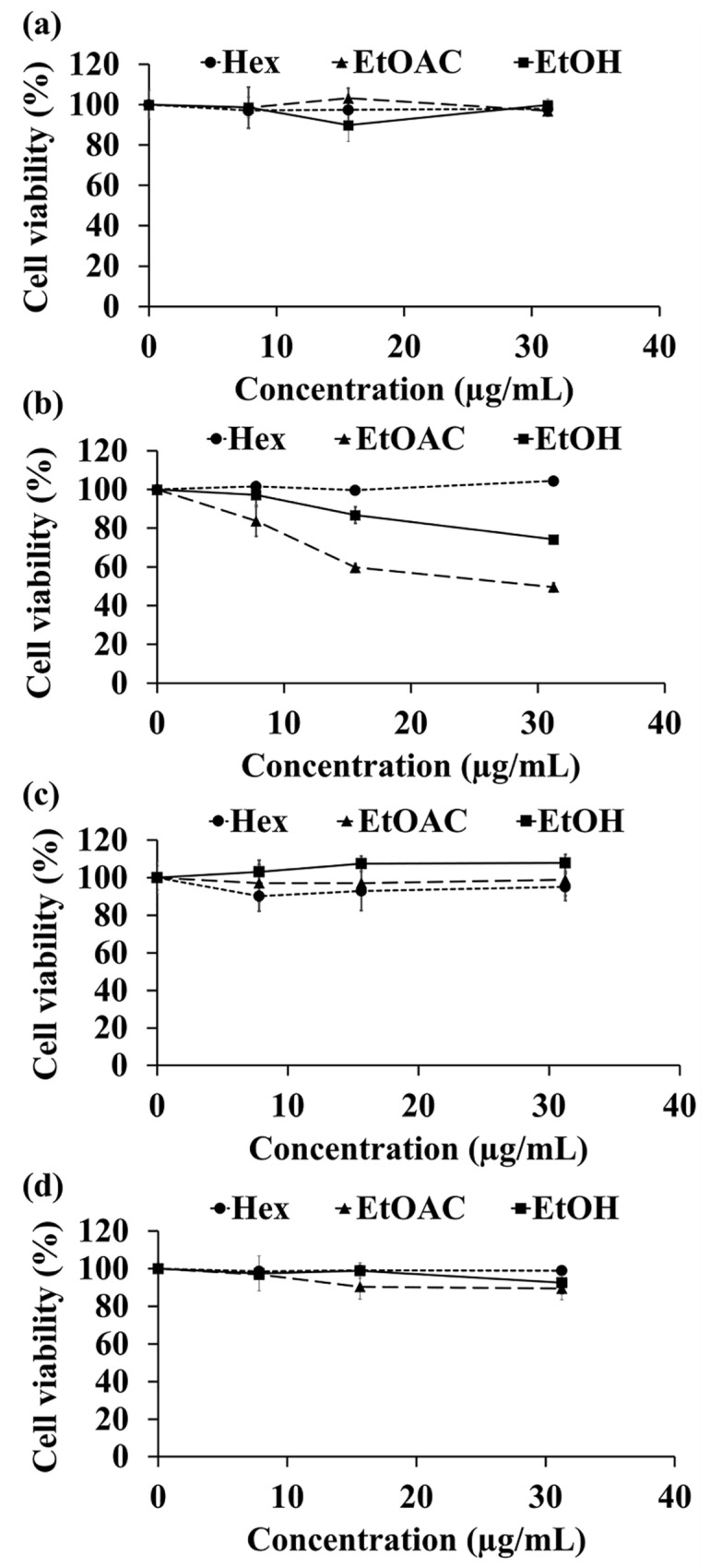
The cytotoxic effect of C. carandas extracts at various concentrations ranging from 0 to 31.25 µg/mL on HaCaT cell line is shown in Figure 9. Except for the extracts from the seed, which were SL and SE, none of the C. carandas extracts inhibited HaCAT cell growth. It would be concluded that leaf, pulp, and fruit of C. carandas had no cytotoxic effect on HaCaT cells.
Figure 9.
Effect of C. carandas extracts from leaf (a), seed (b), pulp (c), and fruit (d) which were sequentially extracted using n-hexane (Hex), ethyl acetate (EtOAc), and 95% ethanol (EtOH), respectively, on viability of HaCaT after treatment for 48 h. The cell viability of HaCaT was determined by MTT colorimetric technique.
4. Conclusions
Ethyl acetate was able to extract the most ursolic acid from different parts of C. carandas, and AL, which had the highest ursolic acid content (411.8 mg/g extract), inhibited MMP-1 activity, NF-κB expression, and tyrosinase activity the most. In contrast, ethanol could extract the greatest amount of phenolics and flavonoids from C. carandas. ES, which yielded the significantly highest phenolic content (GAE = 265.1 ± 1.4 mg/g extract), possessed the significantly highest antioxidant activities with TEAC value of 0.49 ± 0.01 mM/g extract and IC50 against DPPH• of 16.0 ± 0.5 µg/mL (p > 0.05). Furthermore, while EL and ES had comparable ABTS•+ and DPPH• scavenging activity, EL had a higher ferric reducing antioxidant power, with an EC1 of 3.53 ± 0.16 mM/g extract. Inhibition of MMP-2, MMP-9, and elastase was discovered to be the significantly highest in EL. The findings from this study concluded that C. carandas leaves contained the highest level of ursolic acid, phenolics, and flavonoids. However, the solvent used in the extraction process had a significant impact on the chemical compositions and biological activities of C. carandas extracts. Although several C. carandas extracts, such as AL and EL, have biological activities that are beneficial to human skin, AL has been proposed for use as an active ingredient in cosmetics and cosmeceuticals because of its antiwrinkle, anti-inflammation, and whitening properties.
Acknowledgments
W.N. acknowledges the Faculty of Pharmacy, Chiang Mai University, Thailand for the travelling expense to Chia Nan University of Pharmacy and Science, Taiwan.
Author Contributions
Conceptualization, W.N. and W.C.; methodology, W.N., S.A., W.-C.L., S.-C.L., K.-H.L. and W.C.; formal analysis, W.N.; investigation, W.N.; resources, S.A., W.-C.L., S.-C.L., K.-H.L. and W.C.; writing—original draft preparation, W.N. and W.C.; writing—review and editing, W.N. and W.C.; supervision, W.C.; project administration, W.C.; funding acquisition, W.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Graduate School, Chiang Mai University, Thailand and Faculty of Pharmacy, Chiang Mai University, Thailand. The APC was funded by Research Center of Pharmaceutical Nanotechnology, Faculty of Pharmacy, Chiang Mai University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Date is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh A., Uppal G.K. A Review on Carissa carandas-phytochemistry, ethno-pharmacology, and micropropagation as con-servation strategy. Asian J. Pharm. Clin. Res. 2015;8:26–30. [Google Scholar]
- 2.Singh S., Bajpai M., Mishra P. Carissa carandas L. -phyto-pharmacological review. J. Pharm. Pharmacol. 2020;72:1694–1714. doi: 10.1111/jphp.13328. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqui B.S., Ghani U., Ali S.T., Usmani S.B., Begum S. Triterpenoidal Constituents of the Leaves of Carissa Carandas. Nat. Prod. Res. 2003;17:153–158. doi: 10.1080/1478641031000104109. [DOI] [PubMed] [Google Scholar]
- 4.Arif M., Usmani S., Hasan S.M. Bioactive Compounds of Karonda (Carissa carandas L.) In: Murthy H., Bapat V., editors. Bioactive Compounds in Underutilized Fruits and Nuts. Reference Series in Phytochemistry. Springer; Cham, Switzerland: 2019. pp. 1–13. [Google Scholar]
- 5.Tesfaye T., Ravichadran Y.D. Traditional Uses, Pharmacological Action and Phytochemical Analysis of Carissa carandas Linn.: A Review. Nat. Prod. Chem. Res. 2018;6:334. doi: 10.4172/2329-6836.1000334. [DOI] [Google Scholar]
- 6.Bano Z., Begum S., Ali S.S., Kiran Z., Siddiqui B.S., Ahmed A., Khawaja S., Fatima F., Jabeen A. Phytochemicals from Carissa carandas with potent cytotoxic and anti-inflammatory activities. Nat. Prod. Res. 2021:1–6. doi: 10.1080/14786419.2021.1886101. [DOI] [PubMed] [Google Scholar]
- 7.Bosch R., Philips N., Suárez-Pérez J.A., Juarranz A., Devmurari A., Chalensouk-Khaosaat J., González S. Mechanisms of Photoaging and Cutaneous Photocarcinogenesis, and Photoprotective Strategies with Phytochemicals. Antioxidants. 2015;4:248–268. doi: 10.3390/antiox4020248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fusco D., Colloca G., Monaco M.R.L., Cesari M. Effects of antioxidant supplementation on the aging process. Clin. Interv. Aging. 2007;2:377–387. [PMC free article] [PubMed] [Google Scholar]
- 9.El-Domyati M., Attia S., Saleh F., Brown D., Birk D.E., Gasparro F., Ahmad H., Uitto J. Intrinsic aging vs. photoaging: A comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp. Dermatol. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 10.Chaiyana W., Punyoyai C., Somwongin S., Leelapornpisid P., Ingkaninan K., Waranuch N., Srivilai J., Thitipramote N., Wisuitiprot W., Schuster R., et al. Inhibition of 5α-Reductase, IL-6 Secretion, and Oxidation Process of Equisetum debile Roxb. ex Vaucher Extract as Functional Food and Nutraceuticals Ingredients. Nutrients. 2017;9:1105. doi: 10.3390/nu9101105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li H.B., Cheng K.W., Wong C.C., Fan K.W., Chen F., Jiang Y. Evaluation of antioxidant capacity and total phenolic content of different fractions of selected microalgae. Food Chem. 2007;102:771–776. doi: 10.1016/j.foodchem.2006.06.022. [DOI] [Google Scholar]
- 12.Meda A., Lamien C.E., Romito M., Millogo J., Nacoulma O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005;91:571–577. doi: 10.1016/j.foodchem.2004.10.006. [DOI] [Google Scholar]
- 13.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of ‘Antioxidant Power’: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 14.Pellegrini N., Serafini M., Colombi B., Del Rio D., Salvatore S., Bianchi M., Brighenti F. Total antioxidant capacity of plant foods, beverages and oils consumed in Italy assessed by three different in vitro assays. J. Nutr. 2003;133:2812–2819. doi: 10.1093/jn/133.9.2812. [DOI] [PubMed] [Google Scholar]
- 15.Blois M.S. Antioxidant Determinations by the Use of a Stable Free Radical. Nature. 1958;181:1199–1200. doi: 10.1038/1811199a0. [DOI] [Google Scholar]
- 16.Chaiyana W., Anuchapreeda S., Punyoyai C., Neimkhum W., Lee K.-H., Lin W.-C., Lue S.-C., Viernstein H., Mueller M. Ocimum sanctum Linn. as a natural source of skin anti-ageing compounds. Ind. Crop. Prod. 2019;127:217–224. doi: 10.1016/j.indcrop.2018.10.081. [DOI] [Google Scholar]
- 17.Thring T.S., Hili P., Naughton D.P. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009;9:27. doi: 10.1186/1472-6882-9-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee E.K., Lee Y.S., Lee H., Choi C.Y., Park S.H. 14-3-3ε protein increases matrix metalloproteinase-2 gene expression via p38 MAPK signaling in NIH3T3 fibroblast cells. Exp. Mol. Med. 2009;41:453–461. doi: 10.3858/emm.2009.41.7.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bektas N., Şenel B., Yenilmez E., Özatik O., Arslan R. Evaluation of wound healing effect of chitosan-based gel formulation containing vitexin. Saudi Pharm. J. 2020;28:87–94. doi: 10.1016/j.jsps.2019.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grande J., Melder D., Zinsmeister A., Killen P. Transforming growth factor-beta 1 induces collagen IV gene expression in NIH-3T3 cells. Lab. Investig. 1993;69:387–395. [PubMed] [Google Scholar]
- 21.Chiu H.-W., Chen C.-H., Chen Y.-J., Hsu Y.-H. Far-infrared suppresses skin photoaging in ultraviolet B-exposed fibroblasts and hairless mice. PLoS ONE. 2017;12:e0174042. doi: 10.1371/journal.pone.0174042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin F., Zhao H., Yuan X., Zou S., Wang Q., Ma C., Ren Z., Wang Y. In vitro protective effect of ganoderol A isolated from ganaderma lucidum against ultraviolet A radiation and its anti-inflammatory properties. Trop. J. Pharm. Res. 2015;14:415–421. doi: 10.4314/tjpr.v14i3.9. [DOI] [Google Scholar]
- 23.Zhai L., Xu X., Liu J., Jing C., Yang X., Zhao D., Jiang R., Sun L.W. A Novel Biochemical Study of Anti-Dermal Fibroblast Replicative Senescence Potential of Panax Notoginseng Oligosaccharides. Front. Pharmacol. 2021;12:1624. doi: 10.3389/fphar.2021.690538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hong Y.H., Kim D., Nam G., Yoo S., Han S.Y., Jeong S.G., Kim E., Jeong D., Yoon K., Kim S., et al. Photoaging protective effects of BIOGF1K, a compound-K-rich fraction prepared from Panax ginseng. J. Ginseng Res. 2018;42:81–89. doi: 10.1016/j.jgr.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang H., Pan D., Dong Y., Su W., Su H., Wei X., Yang C., Jing L., Tang X., Li X., et al. Transdermal permeation effect of collagen hydrolysates of deer sinew on mouse skin, ex vitro, and antioxidant activity, increased type I collagen secretion of percutaneous proteins in NIH/3T3 cells. J. Cosmet. Dermatol. 2020;19:519–528. doi: 10.1111/jocd.13041. [DOI] [PubMed] [Google Scholar]
- 26.Tomankova K., Kejlova K., Binder S., Daskova A., Zapletalova J., Bendova H., Kolarova H., Jirova D. In vitro cytotoxicity and phototoxicity study of cosmetics colorants. Toxicol. In Vitro. 2011;25:1242–1250. doi: 10.1016/j.tiv.2011.04.026. [DOI] [PubMed] [Google Scholar]
- 27.Meliala D.I.P., Silalahi J., Yuandani Y., Margata L., Satria D. The Role of Coconut Oil to Increase Expression of MMP-9, PDGF-BB, and TGF-β1 in NIH-3T3 Cell Line. Open Access Maced. J. Med. Sci. 2019;7:3733. doi: 10.3889/oamjms.2019.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Binder S., Hanáková A., Tománková K., Pížová K., Bajgar R., Manišová B., Kejlová K., Bendová H., Jírová D., Kolářová H. Adverse phototoxic effect of essential plant oils on NIH 3T3 cell line after UV light exposure. Cent. Eur. J. Public Health. 2016;24:234–240. doi: 10.21101/cejph.a4354. [DOI] [PubMed] [Google Scholar]
- 29.Anuchapreeda S., Fukumori Y., Okonogi S., Ichikawa H. Preparation of Lipid Nanoemulsions Incorporating Curcumin for Cancer Therapy. J. Nanotechnol. 2012:1–11. doi: 10.1155/2012/270383. [DOI] [Google Scholar]
- 30.Chaiyana W., Anuchapreeda S., Leelapornpisid P., Phongpradist R., Viernstein H., Mueller M. Development of micro-emulsion delivery system of essential oil from Zingiber cassumunar Roxb. rhizome for improvement of stability and anti-inflammatory activity. AAPS PharmSciTech. 2017;18:1332–1342. doi: 10.1208/s12249-016-0603-2. [DOI] [PubMed] [Google Scholar]
- 31.Mueller M., Hobiger S., Jungbauer A. Anti-inflammatory activity of extracts from fruits, herbs and spices. Food Chem. 2010;122:987–996. doi: 10.1016/j.foodchem.2010.03.041. [DOI] [Google Scholar]
- 32.Momtaz S., Lall N., Basson A. Inhibitory activities of mushroom tyrosine and DOPA oxidation by plant extracts. S. Afr. J. Bot. 2008;74:577–582. doi: 10.1016/j.sajb.2008.02.005. [DOI] [Google Scholar]
- 33.Curto E.V., Kwong C., Hermersdörfer H., Glatt H., Santis C., Virador V., Hearing V.J., Dooley T.P. Inhibitors of mam-malian melanocyte tyrosinase: In vitro comparisons of alkyl esters of gentisic acid with other putative inhibitors. Biochem. Pharmacol. 1999;57:663–672. doi: 10.1016/S0006-2952(98)00340-2. [DOI] [PubMed] [Google Scholar]
- 34.Nerya O., Vaya J., Musa R., Izrael S., Ben-Arie R., Tamir S. Glabrene and Isoliquiritigenin as Tyrosinase Inhibitors from Licorice Roots. J. Agric. Food Chem. 2003;51:1201–1207. doi: 10.1021/jf020935u. [DOI] [PubMed] [Google Scholar]
- 35.Colombo I., Sangiovanni E., Maggio R., Mattozzi C., Zava S., Corbett Y., Fumagalli M., Carlino C., Corsetto P.A., Scac-cabarozzi D., et al. HaCaT cells as a reliable in vitro differentiation model to dissect the inflammato-ry/repair response of human keratinocytes. Mediat. Inflamm. 2017;2017:7435621. doi: 10.1155/2017/7435621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Siddiqi R., Naz S., Ahmad S., Sayeed S.A. Antimicrobial activity of the polyphenolic fractions derived from Grewia asiatica, Eugenia jambolana and Carissa carandas. Int. J. Food Sci. Technol. 2011;46:250–256. doi: 10.1111/j.1365-2621.2010.02480.x. [DOI] [Google Scholar]
- 37.Azeez S., Karunakaran G., Tripathi P.C., Shivashankara K.S., Roy T.K. Evaluation of antioxidant activity, total phenolics and phytochemical content of selected varieties of karonda fruits (Carissa carandas) Indian J. Agric. Sci. 2016;86:815–822. [Google Scholar]
- 38.Schneider P., Hosseiny S., Szczotka M., Jordan V., Schlitter K. Rapid solubility determination of the triterpenes oleanolic acid and ursolic acid by UV-spectroscopy in different solvents. Phytochem. Lett. 2009;2:85–87. doi: 10.1016/j.phytol.2008.12.004. [DOI] [Google Scholar]
- 39.Fan J.-P., Kong T., Zhang L., Tong S., Tian Z.-Y., Duan Y.-H., Zhang X.-H. Solubilities of Ursolic Acid and Oleanolic Acid in Four Solvents from (283.2 to 329.7) K. J. Chem. Eng. Data. 2011;56:2723–2725. doi: 10.1021/je101309a. [DOI] [Google Scholar]
- 40.Mlala S., Oyedeji A.O., Gondwe M., Oyedeji O.O. Ursolic Acid and Its Derivatives as Bioactive Agents. Molecules. 2019;24:2751. doi: 10.3390/molecules24152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhar G., Akther S., Sultana A., May U., Islam M.M., Dhali M., Sikdar D. Effect of extraction solvents on phenolic con-tents and antioxidant capacities of Artocarpus chaplasha and Carissa carandas fruits from Bangladesh. J. Appl. Biol. Biotech. 2017;5:39–44. [Google Scholar]
- 42.Verma K., Shrivastava D., Kumar G. Antioxidant activity and DNA damage inhibition in vitro by a methanolic extract of Carissa carandas (Apocynaceae) leaves. J. Taibah Univ. Sci. 2015;9:34–40. doi: 10.1016/j.jtusci.2014.07.001. [DOI] [Google Scholar]
- 43.Patil R.P., Pai S.R., Pawar N.V., Shimpale V.B., Patil R.M., Nimbalkar M.S. Chemical characterization, mineral analysis, and antioxidant potential of two underutilized berries (Carissa carandus and Eleagnus conferta) from the Western Ghats of India. Crit. Rev. Food Sci. Nutr. 2012;52:312–320. doi: 10.1080/10408398.2010.500227. [DOI] [PubMed] [Google Scholar]
- 44.Bissett D.L., Oblong J.E., Berge C.A. Niacinamide: AB vitamin that improves aging facial skin appearance. Dermatol. Surg. 2005;31:860–866. doi: 10.1111/j.1524-4725.2005.31732. [DOI] [PubMed] [Google Scholar]
- 45.Kawada A., Konishi N., Oiso N., Kawara S., Date A. Evaluation of anti-wrinkle effects of a novel cosmetic containing niacinamide. J. Dermatol. 2008;35:637–642. doi: 10.1111/j.1346-8138.2008.00537.x. [DOI] [PubMed] [Google Scholar]
- 46.Humbert P.G., Haftek M., Creidi P., Lapière C., Nusgens B., Richard A., Schmitt D., Rougier A., Zahouani H. Topical ascorbic acid on photoaged skin. Clinical, topographical and ultrastructural evaluation: Double-blind study vs. placebo. Exp. Dermatol. 2003;12:237–244. doi: 10.1034/j.1600-0625.2003.00008.x. [DOI] [PubMed] [Google Scholar]
- 47.Kohl E., Steinbauer J., Landthaler M., Szeimies R.M. Skin ageing. J. Eur. Acad. Dermatol. Venereol. 2011;25:873–884. doi: 10.1111/j.1468-3083.2010.03963.x. [DOI] [PubMed] [Google Scholar]
- 48.Kähäri V.-M., Saarialho-Kere U. Matrix metalloproteinases in skin. Exp. Dermatol. 1997;6:199–213. doi: 10.1111/j.1600-0625.1997.tb00164.x. [DOI] [PubMed] [Google Scholar]
- 49.Pillai S., Oresajo C., Hayward J. Ultraviolet radiation and skin aging: Roles of reactive oxygen species, inflammation and protease activation, and strategies for prevention of inflammation-induced matrix degradation-a review. Int. J. Cosmet. Sci. 2005;27:17–34. doi: 10.1111/j.1467-2494.2004.00241.x. [DOI] [PubMed] [Google Scholar]
- 50.Kandasamy A.D., Chow A.K., Ali M.A., Schulz R. Matrix metalloproteinase-2 and myocardial oxidative stress injury: Beyond the matrix. Cardiovasc. Res. 2010;85:413–423. doi: 10.1093/cvr/cvp268. [DOI] [PubMed] [Google Scholar]
- 51.Ricci S., D’Esposito V., Formisano P., Di Carlo A. Substrate-zymography: A still worthwhile method for gelatinases analysis in biological samples. Clin. Chem. Lab. Med. 2016;54:1281–1290. doi: 10.1515/cclm-2015-0668. [DOI] [PubMed] [Google Scholar]
- 52.Tentes I., Asimakopoulos B., Mourvati E., Diedrich K., Al-Hasani S., Nikolettos N. Matrix metalloproteinase (MMP)-2 and MMP-9 in seminal plasma. J. Assist. Reprod. Genet. 2007;24:278–281. doi: 10.1007/s10815-007-9129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Visse R., Nagase H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochem-istry. Circ. Res. 2003;92:827–839. doi: 10.1161/01.RES.0000070112.80711.3D. [DOI] [PubMed] [Google Scholar]
- 54.Jabłońska-Trypuć A., Matejczyk M., Rosochacki S. Matrix metalloproteinases (MMPs), the main extracellular matrix (ECM) enzymes in collagen degradation, as a target for anticancer drugs. J. Enzym. Inhib. Med. Chem. 2016;31:177–183. doi: 10.3109/14756366.2016.1161620. [DOI] [PubMed] [Google Scholar]
- 55.Kim J.-A., Ahn B.-N., Kong C.-S., Kim S.-K. Protective effect of chromene isolated from Sargassum horneri against UV-A-induced damage in skin dermal fibroblasts. Exp. Dermatol. 2012;21:630–631. doi: 10.1111/j.1600-0625.2012.01535.x. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji N., Moriwaki S., Suzuki Y., Takema Y., Imokawa G. The Role of Elastases Secreted by Fibroblasts in Wrinkle Formation: Implication Through Selective Inhibition of Elastase Activity. Photochem. Photobiol. 2007;74:283–290. doi: 10.1562/0031-8655(2001)0740283TROESB2.0.CO2. [DOI] [PubMed] [Google Scholar]
- 57.Kim S.Y., Go K.C., Song Y.S., Jeong Y.S., Kim E.J., Kim B.J. Extract of the mycelium of T. matsutake inhibits elastase activi-ty and TPA-induced MMP-1 expression in human fibroblasts. Int. J. Mol. Med. 2014;34:1613–1621. doi: 10.3892/ijmm.2014.1969. [DOI] [PubMed] [Google Scholar]
- 58.Ying Q.L., Rinehart A.R., Simon S.R., Cheronis J.C. Inhibition of human leucocyte elastase by ursolic acid. Evidence for a binding site for pentacyclic triterpenes. Biochem. J. 1991;277:521–526. doi: 10.1042/bj2770521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee K.K., Cho J.J., Park E.J., Choi J.D. Anti-elastase and anti-hyaluronidase of phenolic substance from Areca catechuas a new anti-ageing agent. Int. J. Cosmet. Sci. 2001;23:341–346. doi: 10.1046/j.0412-5463.2001.00102.x. [DOI] [PubMed] [Google Scholar]
- 60.Abdullah N.H., Thomas N.F., Sivasothy Y., Lee V.S., Liew S.Y., Noorbatcha I.A., Awang K. Hyaluronidase Inhibitory Activity of Pentacylic Triterpenoids from Prismatomeris tetrandra (Roxb.) K. Schum: Isolation, Synthesis and QSAR Study. Int. J. Mol. Sci. 2016;17:143. doi: 10.3390/ijms17020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Buhren B.A., Schrumpf H., Hoff N.P., Bölke E., Hilton S., Gerber P.A. Hyaluronidase: From clinical applications to molec-ular and cellular mechanisms. Eur. J. Med. Res. 2016;21:1–7. doi: 10.1186/s40001-016-0201-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tammi R., Ripellino J.A., Margolis R.U., Tammi M. Localization of epidermal hyaluronic acid using the hyaluronate bind-ing region of cartilage proteoglycan as a specific probe. J. Investig. Dermatol. 1988;90:412–414. doi: 10.1111/1523-1747.ep12456530. [DOI] [PubMed] [Google Scholar]
- 63.Kuppusamy U., Khoo H., Das N. Structure-activity studies of flavonoids as inhibitors of hyaluronidase. Biochem. Pharmacol. 1990;40:397–401. doi: 10.1016/0006-2952(90)90709-T. [DOI] [PubMed] [Google Scholar]
- 64.Zeng H.-J., Ma J., Yang R., Jing Y., Qu L.-B. Molecular Interactions of Flavonoids to Hyaluronidase: Insights from Spectroscopic and Molecular Modeling Studies. J. Fluoresc. 2015;25:941–959. doi: 10.1007/s10895-015-1576-3. [DOI] [PubMed] [Google Scholar]
- 65.Medzhitov R. Origin and physiological roles of inflammation. Nat. Cell Biol. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- 66.Tian F., Zhou P., Kang W., Luo L., Fan X., Yan J., Liang H. The small-molecule inhibitor selectivity between IKKα and IKKβ kinases in NF-κB signaling pathway. J. Recept. Signal Transduct. Res. 2015;35:307–318. doi: 10.3109/10799893.2014.980950. [DOI] [PubMed] [Google Scholar]
- 67.Chen L., Deng H., Cui H., Fang J., Zuo Z., Deng J., Li Y., Wang X., Zhao L. Inflammatory responses and inflamma-tion-associated diseases in organs. Oncotarget. 2017;9:7204. doi: 10.18632/oncotarget.23208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kany S., Vollrath J.T., Relja B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019;20:6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li R., Hong P., Zheng X. β-carotene attenuates lipopolysaccharide-induced inflammation via inhibition of the NF-κB, JAK2/STAT3 and JNK/p38 MAPK signaling pathways in macrophages. Anim. Sci. J. 2018;90:140–148. doi: 10.1111/asj.13108. [DOI] [PubMed] [Google Scholar]
- 70.Matsusaka T., Fujikawa K., Nishio Y., Mukaida N., Matsushima K., Kishimoto T., Akira S. Transcription factors NF-IL6 and NF-kappa B synergistically activate transcription of the inflammatory cytokines, interleukin 6 and interleukin 8. Proc. Natl. Acad. Sci. USA. 1993;90:10193–10197. doi: 10.1073/pnas.90.21.10193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun H., Cai W., Wang X., Liu Y., Hou B., Zhu X., Qiu L. Vaccaria hypaphorine alleviates lipopolysaccharide-induced inflammation via inactivation of NFκB and ERK pathways in Raw 264.7 cells. BMC Complement. Altern. Med. 2017;17:120. doi: 10.1186/s12906-017-1635-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z., Jiang W., Zhang Z., Qian M., Du B. Nitidine chloride inhibits LPS-induced inflammatory cytokines production via MAPK and NF-kappaB pathway in RAW 264.7 cells. J. Ethnopharmacol. 2012;144:145–150. doi: 10.1016/j.jep.2012.08.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Date is contained within the article.