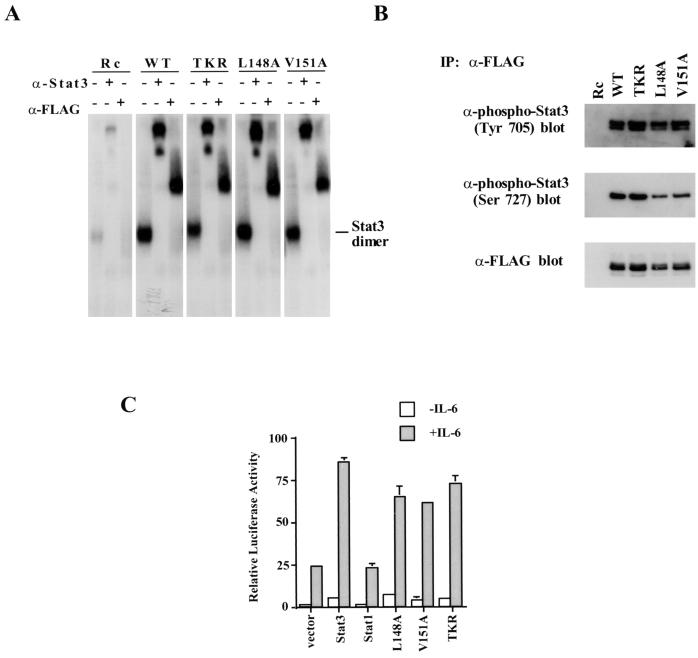
FIG. 7.
The noninteractive Stat3 mutants can bind DNA and activate IL-6-dependent transcription. (A) The DNA binding abilities of three noninteractive Stat3 mutants were examined by gel mobility shift analysis with 32P-labeled M67 probe. 293T cells were transiently transfected with either wild-type (WT) Stat3 or mutant Stat3 cDNAs treated with IL-6 at a concentration of 5 ng/ml and recombinant human IL-6 soluble receptor at a concentration of 5 ng/ml for 30 min. Nuclear extracts were prepared from these cells, and 3 mg of extract was used in each EMSA. Rc, pRcCMV; α-Stat3, anti-Stat3; α-FLAG, anti-FLAG. (B) Phosphorylation on tyrosine and serine residues of the three Stat3 mutants was indistinguishable from that of wild-type Stat3. Nuclear extracts (75 μ) from transfected 293T cells were immunoprecipitated (IP) with anti-FLAG antibody, and the immunoprecipitates were then subjected to SDS–7% PAGE followed by Western blotting with the antibodies indicated. (C) The IL-6-dependent transcriptional activities of three Stat3 mutants were examined by using the 3xLy6E luciferase reporter. −, no IL-6 added; +, IL-6 present.