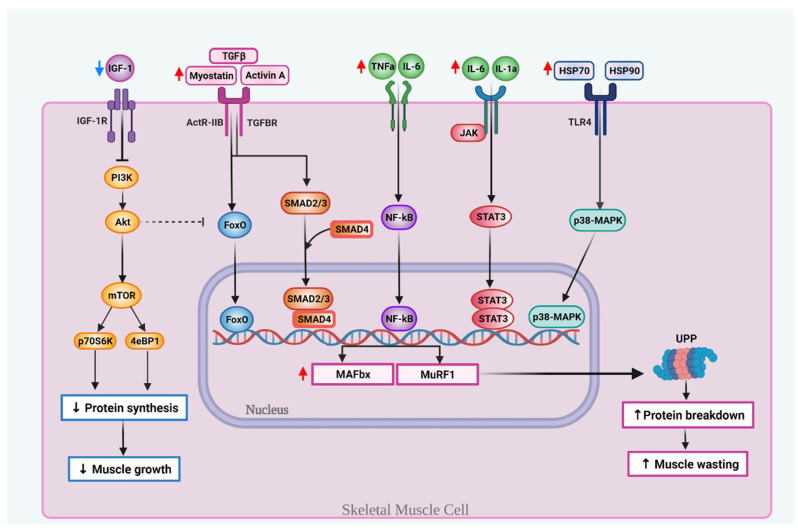
Figure 1.
Key molecular mechanisms involved in muscle wasting during PDAC-associated cachexia. Cachexia is associated with decreased levels of insulin-like growth factor 1 (IGF-1), which inhibits protein synthesis, in part, by suppressing the PI3K-Akt-mTOR pathway. Transforming growth factor-β (TGF-β) family members, like myostatin and activin A, bind to the ActRIIB receptor complex or TGFβ receptor and activate the forkhead (FoxO) family transcription factor or Smad2/3. Smad2/3 makes a complex with Smad4. Pro-inflammatory cytokines, like tumor necrosis factor α (TNF-α), interleukin 6 (IL-6) and IL-1a can activate the nuclear factor kappa-light-chain enhancer of B cells (NF-κB) and/or the janus tyrosine kinase/signal transducer and activation of transcription (JAK/STAT). The tumor releases surface heat shock proteins Hsp70 and Hsp90 that activate the Toll-like receptor (TLR4) and upregulates p38-mitogen activated protein kinase (MAPK). The translocation to the nucleus of FoxO, SMAD2/3-4 complex, NF-κB, STAT3 or p38-MAPK induces the subsequent transcription of the muscle atrophy F-box protein (MAFBX) and muscle RING finger-containing protein 1 (MURF1), two genes that activate the ubiquitin-proteasome pathway (UPP) and induce proteolysis, which in turn increases and induces muscle-wasting.