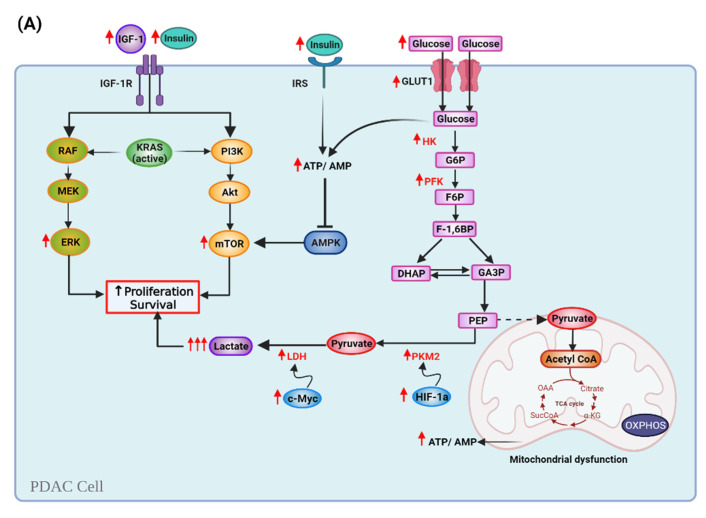
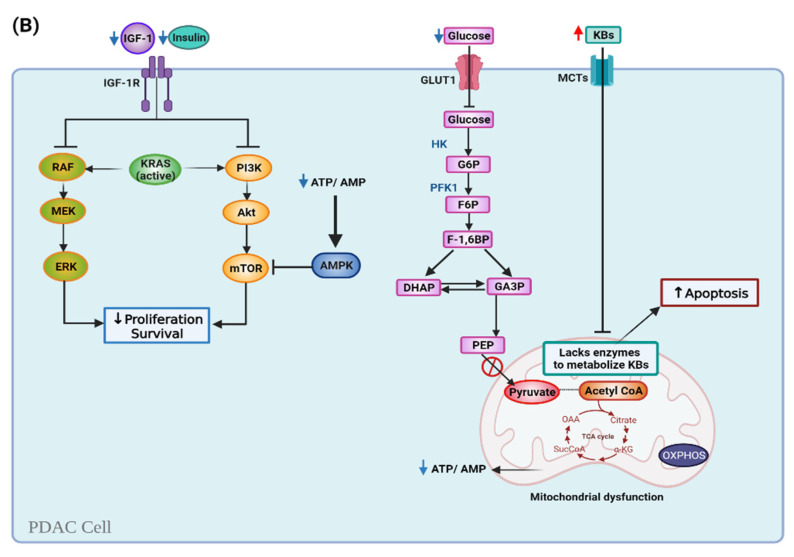
Figure 3.
Pancreatic cancer cell metabolism during a normal carbohydrate-rich diet versus a ketogenic diet. (A) Cancer cells undergo various metabolic modifications to satisfy their energy needs. High levels of insulin and insulin-like growth factor-I levels (IGF) induce upregulation of insulin/IGF-1-dependent phosphatidylinositol 3-kinase (PI3K)/Akt/mammalian target of the rapamycin (mTOR) system and the Ras/Raf/Mitogen-activated protein kinase/ERK kinase (MEK)/extracellular-signal-regulated kinase (ERK) cascade. KRAS mutation affects glucose dependency. The expression of glucose transporter 1 (GLUT1) is stimulated, increasing glucose uptake and glycolysis. Pyruvate kinase isoform M2 (PKM2) and lactate dehydrogenase (LDH) are over-expressed, so lactate levels increase. Hyperglycemia inhibits AMP-activated protein kinase (AMPK), which in term activates mTOR (B) KDs reduce circulating glucose, which halts glycolysis. Decreased blood glucose, insulin, and IGF-I levels inhibit the PI3K/Akt/mTOR pathway, and lactate production, therefore inducing selective starvation in cancer cells, and targeting proliferation and survival. Ketosis activates AMPK, which inhibits mTOR. Mitochondrial dysfunction and lack of the mitochondrial enzymes that metabolize ketone bodies (KBs) cause the mitochondria to decrease ATP production. Note: Upward red arrows mean upregulation; downward blue arrows mean downregulation.