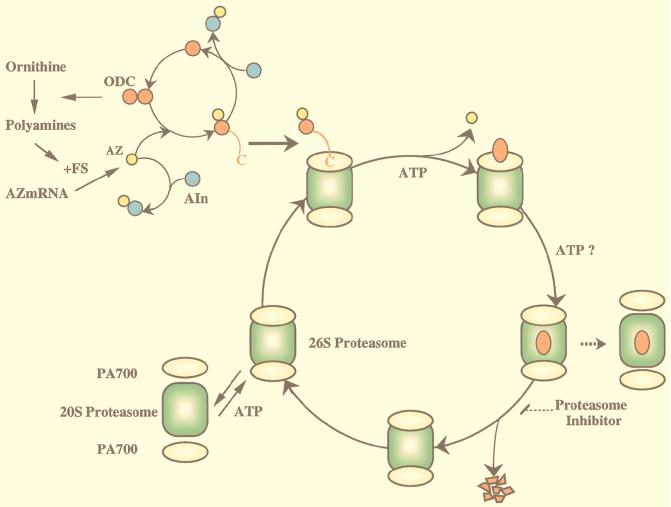
FIG. 10.
Model of ODC degradation by the 26S proteasome. ODC is active as a homodimer complex. AZ, which is induced by translational frameshifting, binds to inactive monomeric ODC to form an ODC-AZ complex. Two terminal regulatory subcomplexes, termed PA700, are attached in an ATP-dependent manner to both ends of the 20S proteasome (central catalytic machinery) in opposite orientations to form enzymatically active proteasomes. The 26S proteasome may attack the exposed ODC C-terminal region and pull the ODC molecule, but not AZ, into the interior, which is associated with ATP-dependent substrate unfolding. The continuous translocation of unfolded ODC (inactivated ODC) may be required for further continuous unfolding of ODC (ODC inactivation), and the two processes may proceed in concert with each other. The unfolded ODC translocated into the cavity of the 20S proteasome, which harbors proteolytically active sites, is degraded, but it is unknown whether ATP consumption is needed for the degradative process itself. After degradation, the 26S proteasome traps another substrate(s) or may be in part dissociated into its constituents, the 20S proteasome and PA700. See the text for details.