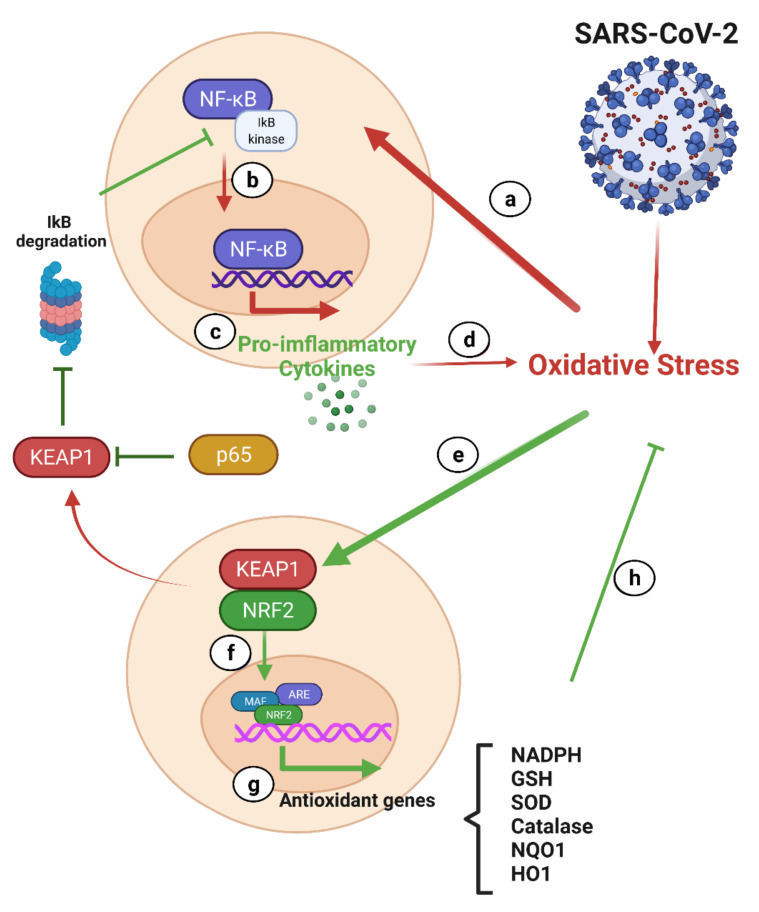
Figure 6.
Crosstalk between NF-κB and Nrf2 signaling in COVID-19 [226]. In uninfected cells, the NF-κB subunit is limited to the cytoplasm because of the inhibitory effect of the κB inhibitor family (IκB). (a) SARS-CoV-2 infection can increase oxidative stress and cause IKβ kinase activation, which causes phosphorylation of IkB-α (an NF-κB inhibitor), leading to proteasomal degradation of IkB-α and subsequent release of NF-κB. (b) In infected cells, NF-κB containing p65 (a NF-kB subunit; KEAP1 inhibitor) is transferred to the nucleus and acts on the DNA response elements. (c) This leads to the transcription of numerous pro-inflammatory cytokines. (d) The activated NF-κB signaling cascade produces excessive pro-inflammatory cytokines and exacerbates the oxidative state. (e) In contrast, oxidative stress elicits Nrf2 signaling, leading to the separation of Nrf2 from its inhibitor Keap1. (f) Nrf2 then moves to the nucleus and binds to the Maf protein and the antioxidant response element (ARE). (g) Activated ARE transcripts include antioxidant genes and phase II enzymes such as NADPH, GSH, SOD, catalase, heme oxygenase-1, and NQO1. All these enzymes enhance the degradation of ROS. In addition, free Keap1 can prevent the degradation of IkB-α. (h) In general, genetic intervention and subsequent transcription have a positive effect of the Nrf2 pathway in the reduction of oxidative stress. Infection with COVID-19 causes oxidative stress, which also causes antioxidant benefits associated with Nrf2 [226]. Figure generated with Biorender (https://biorender.com/accessed on 6 September 2021).