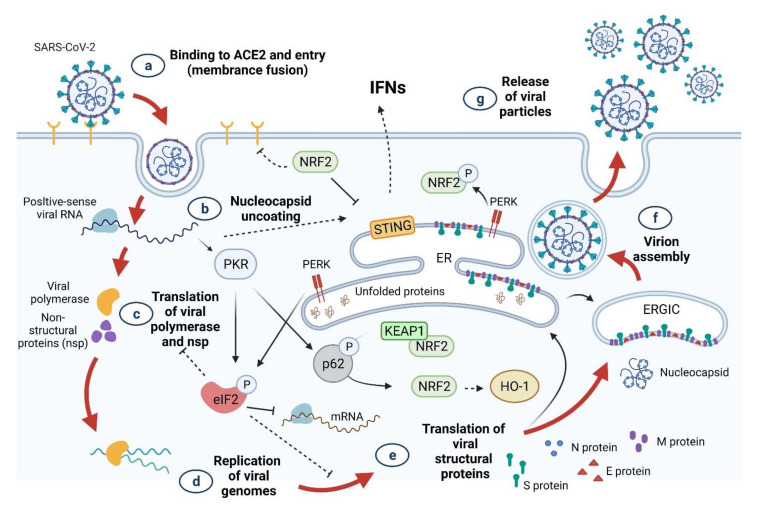
Figure 8.
Potential crosstalk between the SARS-CoV-2 viral cycle and the RSV-mediated induction of Nrf2 activity. RSV supplementation activates the transcriptional activity of Nrf2, which can interface with the SARS-CoV-2 viral cycle. This figure shows the various stages of the viral cycle that could be influenced by Nrf2 signaling. (a) Binding of the viral spike protein (S) to ACE2 results in virion entry. Nrf2 suppresses the expression of the ACE2 gene [262]. (b) The viral nucleocapsid is uncoated in the cytoplasm of infected cells. (c) Next, the translation of viral +ssRNA and division of the products into different viral proteins occurs. Moreover, viral RNA within the host cell activates the cGAS DNA/RNA sensor, which transmits signals through the STING adapter [263], and facilitates the expression of type I and III IFNs [264]. Nrf2 suppresses IFN production by reducing STING expression [265]. (d) With respect to the replication of the SARS-CoV-2 genome, Nrf2 promotes HO-1 expression, generating Fe2+ that can bind to the divalent metal-binding pocket of the RdRp of SARS-CoV-2 and inhibits its catalytic activity [266]. (e) To allow for the translation of viral structural proteins, double-stranded RNA-activated PKR phosphorylates eIF2 and inhibits the translation of viral proteins. PKR also phosphorylates p62 and activates Nrf2 when its suppressor, KEAP1, is removed by autophagy [267]. Nrf2 is a PERK substrate that acts as a PERK-dependent cell-survival effector. Inhibition of viral proteins also activates the UPR. PERK is a vital Ser/Thr kinase protein that also plays a role in the UPR pathway. Nrf2 phosphorylation leads to the stabilization and improvement of its transcriptional activity [268]. (f) Virion assembly. Autophagy acts as a cell-monitoring mechanism for the control of invasive pathogens. SARS-CoV-2 ORF3a inhibits autophagy activity by blocking the formation of autolysosomes, which may destroy the newly synthesized virion [269]. The newly formed viral particles are assembled across the endoplasmic reticulum and the Golgi complex. (g) Release of viral particles. Structural proteins play an important role in the budding of viral particles released by infected host cells [270]. Numerous studies have proposed that ACE2 receptors are key structural proteins for virus budding and entry into host cells [271]. ACE2: angiotensin-converting enzyme 2; eIF2: eukaryotic initiation factor 2; ER: endoplasmic reticulum; ER: Golgi intermediate compartment; HO-1: heme oxygenase 1; IFN: interferon; KEAP1: Kelch-like ECH-associated protein 1; NRF2: nuclear factor erythroid 2 p45-related factor 2; PERK: PKR-like endoplasmic reticulum kinase; P: phosphorylation; PKR: protein kinase R; STING: stimulator of interferon genes; RdRp, RNA-dependent RNA polymerase; UPR, unfolded protein response. Figure generated with Biorender (https://biorender.com/accessed on 6 September 2021).