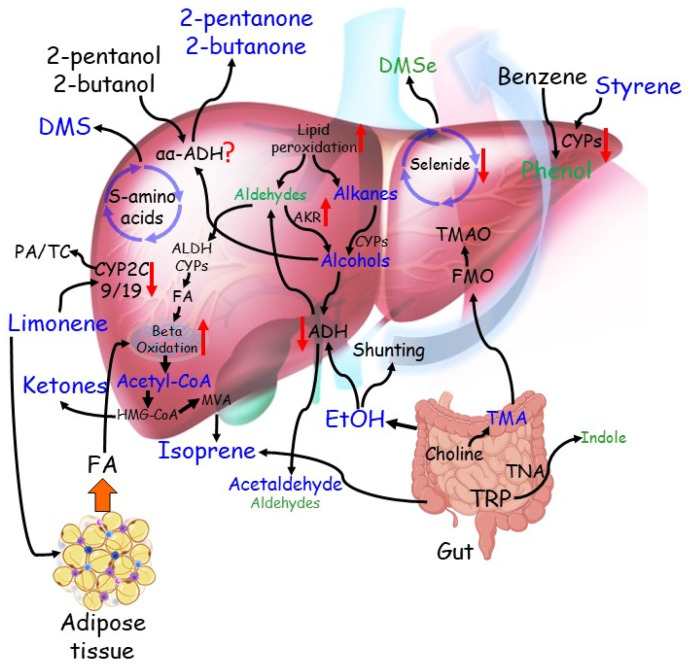
Figure 2.
The complex network of established and proposed metabolic pathways from which VOCs stem and their alterations in chronic liver diseases. Compounds found elevated in the breath of patients with cirrhosis are indicated in blue, those downregulated in green. Red arrows indicate changes in the metabolic pathways. From the bottom left: insulin resistance increases fatty acid (FA) shuttling from the adipose tissue to the liver. The resulting excess of acetyl-CoA is metabolised in the mevalonate pathway (MVA) to ketones and isoprene, the latter also generates from gut microbiota. Dietary limonene is converted to Perillyl alcohol (PA) and trans-Carveol (TC) mainly by CYP2C9 and CYP2C19. PA and TC are more soluble in the aqueous environment and can be excreted in urine. In the cirrhotic liver, reduced activity of CYP enzymes leads to the accumulation of limonene in the adipose tissue and increases its permanence in the body, resulting in elevated levels in the breath. Incomplete metabolism of sulphur-containing amino acids in the transamination pathway, coupled with Cytochrome C oxidase deficiency in the cirrhotic liver, lead to elevated levels of Dimethyl-sulphide (DMS) in the breath of patients with cirrhosis. Dietary 2-butanol, a flavouring agent, and a compound contained in fruit is converted to 2-butanone by αα-ADH. A similar pathway may also involve 2-pentanol, a similar compound. Both 2-butanone and 2-pentanone have been found elevated in the breath of patients with cirrhosis. Lipid peroxidation, a process triggered by increased inflammation of the cirrhotic liver, has been proposed to generate alkanes, such as octane, pentane and ethane, and medium, long-chain aldehydes. These alkanes have been found elevated, while detected aldehydes are reduced. Both classes of compounds can be converted to corresponding alcohols by CYPs or aldo-keto reductases (AKR), respectively. Medium-chain primary alcohols can be further metabolised by alcohol dehydrogenases (ADH) back to aldehydes, which can be converted to corresponding fatty acids and feed beta-oxidation. Secondary alcohols such as 2-butanol and 2-butanone may also be generated and contribute to increasing the corresponding ketones. Ethanol (ETOH), which originates from the diet, sugar fermentation from gut microbiota, and oxidation of ethane, increases in the breath of patients with cirrhosis because of shunting and downregulation of the main metabolising pathway. However, acetaldehyde, the main bio-product of ETOH metabolism, has also been elevated due to downregulation of the downstream enzyme aldehyde dehydrogenase (ALDH). Dimethyl selenide (DMSe) is one of the excretion products of selenide metabolism. Selenide blood levels were reduced in patients with cirrhosis, to an extent related to disease severity. Therefore, reduced DMSe in breath may result from a lack of substrate and impaired selenide metabolic pathway. Benzene is a pollutant generated mainly by petrol products and readily adsorbed by the body by inhalation. Benzene is oxidised to phenol by the CYP system. Reduced CYP activity in cirrhosis may explain reduced breath levels of phenol. Exposure to styrene takes place mainly by adsorption of vapours through the lungs. Its reduced oxidation by the CYP system explains its increase in the breath of patients with cirrhosis. Trimethylamine (TMA) is derived from the diet by microbial degradation of precursors such as choline. TMA is readily absorbed and metabolised by flavin-containing monooxygenases (FMO) in trimethylamine N-oxide (TMAO) for urine excretion. Reduced FMO activity in cirrhosis may lead to increased TMA in the breath. Indole is a catabolic product of tryptophane (TRP) metabolism by tryptophanase (TNA) activity of gut microbiota, which alterations in cirrhosis may lead to reduced indole exhalation in the breath.