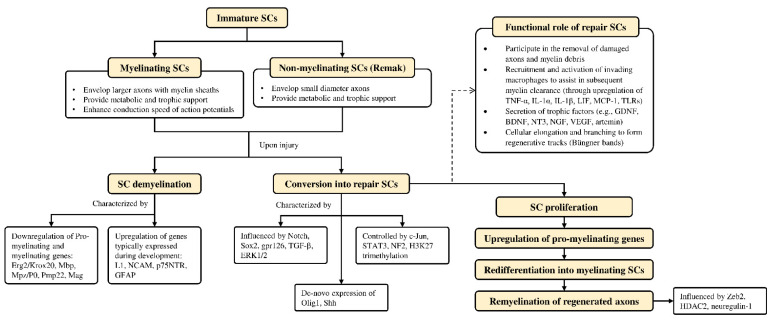
Figure 3.
Overview of Schwann cell plasticity and their roles following peripheral nerve injury. Immature SCs develop into either myelinated or non-myelinated forms depending on the type of axon association. Upon nerve injury, SCs are capable of converting into a repair phenotype alongside the demyelination process that is mediated by different genes and transcriptional mechanisms. These events promote neuronal survival and enhance axonal regeneration following injury. Subsequently, repair SCs can be reprogrammed back to remyelinate regenerated axons. Further details on SC plasticity are presented in the reviews by Jessen & Mirsky [39] and Nocera & Jacob [100]. BDNF—brain-derived neurotrophic factor; Erg2/Krox20—early growth response 2; ERK—extracellular signal-regulated protein kinase; GDNF—glial cell-derived neurotrophic factor; GFAP—glial fibrillary acidic protein; gpr126—adhesion G protein-coupled receptor G6; H3K27—methylation of histone H3 on lysine 27; HDAC2—histone deacetylase 2; IL—interleukin; L1—L1 cell adhesion molecule; LIF—leukemia inhibitory factor; Mag—myelin associated glycoprotein; Mbp—myelin basic protein; MCP-1—monocyte chemotactic protein 1; Mpz/P0—myelin protein zero; NCAM—neural cell adhesion molecule; NF2—neurofibromatosis 2; NGF—nerve growth factor; NT3—neurotrophin-3; Olig1—oligodendrocyte transcription factor 1; p75NTR—p75 neurotrophin receptor; Pmp22—peripheral myelin protein 22; SCs—Schwann cells; Shh—Sonic Hedgehog; Sox2—(sex determining region Y)-box 2; STAT3—signal transducer and activator of transcription 3; TGF-β—transforming growth factor-β; TLRs—Toll-like receptors; TNF-α—tumor necrosis factor-α; VEGF—vascular endothelial growth factor; Zeb2—zinc finger E-box-binding homeobox 2.