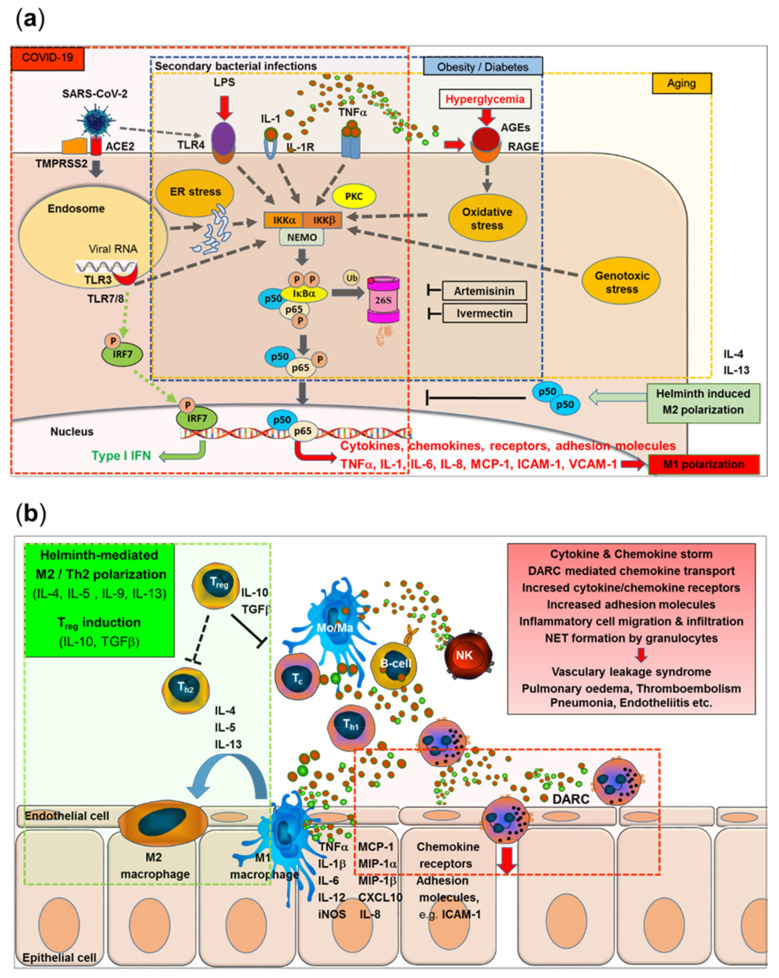
Figure 5.
(a). SARS-CoV-2 binds to the ACE-2 receptor, followed by spike protein cleavage, membrane fusion, and endocytosis. RNA of single-stranded RNA viruses activates the Toll-like receptors TLR7 and TLR8. Double-stranded RNA intermediates are recognized by TLR3. TLR activation triggers transcription of the interferon-regulator factor (IRF) family, but also the activation of IKK (IκB kinases), phosphorylation, ubiquitination, and proteasomal degradation of the cytoplasmic inhibitor factor IκBα, and release of the NF-κB (p50/p65) to enter the nucleus. The final sequence of NF-κB activation is shared with multiple cytokine receptor- and TLR-mediated signal cascades, e.g., binding of TNFα or IL-1 to their receptors or binding of LPS to TLR4. Additionally, SARS-CoV-2 leads to TLR4-mediated and ER stress-induced NF-κB activation. NF-κB activation triggers gene expression for multiple pro-inflammatory cytokines and chemokines (with a typical M1/Th1 polarization pattern), adhesion molecules, and acute phase proteins. Released cytokines feed into accelerating feedback loops. In contrast, interferon-response factor (IRF)-related responses are largely independent of NF-κB translocation (a, red transparent area). Metabolic disorders, e.g., obesity and diabetes, lead to chronic NF-κB pre-activation via hyperglycemia-induced IKKβ overexpression, Protein kinase C (PKC) activation, and advanced glycosylation end products (AGEs) that bind to receptors for advanced glycosylation end products (RAGE) on vascular smooth muscle cells, leading to oxidant stress. NF-κB triggered cytokine (TNFα, IL-1β) expression feeds into accelerating feedback loops via TNFα/IL-1β receptor-triggered NF-κB activation and increased expression of RAGE (a, blue transparent area). Aging-related genotoxicity, inflammation, ER stress, and oxidative stress stimulate the NF-κB pathway. An “inflamm-aging”-associated dysregulation of cytokine homeostasis feeds into auto amplifying loops, enhancing the COVID-19-triggered acute NF-κB activation storm (a, yellow transparent area). The anti-helminth drug ivermectin and the anti-malaria drug artemisinin inhibit the NF-κB pathway at various steps, with artemisinin (a proteasome inhibitor) blocking the degradation of IκBα and the nuclear translocation of NF-κB. (b). Helminth infection induces M2/Th2 predisposition, Treg, and immunosuppressive cytokines IL-10 and TGFβ (b, green transparent area), which can counteract SARS-CoV-2-triggered NF-κB-mediated M1/Th1 polarization (a,b, green inserts). Duffy receptor-mediated transport and transcytosis of chemokines can enhance the migration, adhesion, and extravasation of macrophages and neutrophils even at sites distant to the initially SARS-CoV-2-infected cells (b, red transparent area), leading to the activation and damage of endothelial cells, vascular leakage, pulmonary edema, thromboembolism, and multiorgan endotheliitis (b, red insert). This effect may be reduced in geographical areas with prevalent DARC negativity. (a) represents a modification of Figure 1 previously published in https://pubmed.ncbi.nlm.nih.gov/33362782/ accessed on 8 September 2021.