Abstract
Some enteric parasites causing zoonotic diseases in livestock have been poorly studied or even neglected. This is the case in stramenopile Blastocystis sp. and the microsporidia Enterocytozoon bieneusi in Spain. This transversal molecular epidemiological survey aims to estimate the prevalence and molecular diversity of Blastocystis sp. and E. bieneusi in cattle faecal samples (n = 336) in the province of Álava, Northern Spain. Initial detection of Blastocystis and E. bieneusi was carried out by polymerase chain reaction (PCR) and Sanger sequencing of the small subunit (ssu) rRNA gene and internal transcribed spacer (ITS) region, respectively. Intra-host Blastocystis subtype diversity was further investigated by next generation amplicon sequencing (NGS) of the ssu rRNA gene in those samples that tested positive by conventional PCR. Amplicons compatible with Blastocystis sp. and E. bieneusi were observed in 32.1% (108/336, 95% CI: 27.2–37.4%) and 0.6% (2/336, 95% CI: 0.0–1.4%) of the cattle faecal samples examined, respectively. Sanger sequencing produced ambiguous/unreadable sequence data for most of the Blastocystis isolates sequenced. NGS allowed the identification of 10 Blastocystis subtypes including ST1, ST3, ST5, ST10, ST14, ST21, ST23, ST24, ST25, and ST26. All Blastocystis-positive isolates involved mixed infections of 2–8 STs in a total of 31 different combinations. The two E. bieneusi sequences were confirmed as potentially zoonotic genotype BEB4. Our data demonstrate that Blastocystis mixed subtype infections are extremely frequent in cattle in the study area. NGS was particularly suited to discern underrepresented subtypes or mixed subtype infections that were undetectable or unreadable by Sanger sequencing. The presence of zoonotic Blastocystis ST1, ST3, and ST5, and E. bieneusi BEB4 suggest cross-species transmission and a potential risk of human infection/colonization.
Keywords: Blastocystis, Enterocytozoon bieneusi, cattle, NGS, ribosomal RNA, subtypes, Spain, zoonoses, transmission
1. Introduction
Some enteric parasites causing zoonotic diseases in livestock in Spain have been poorly studied or even neglected. This is the case in stramenopile Blastocystis sp. and the microsporidia Enterocytozoon bieneusi. Blastocystis sp. is probably the most common enteric parasite in humans, affecting more than 1 billion people globally [1]. Human Blastocystis infection has been associated with a variety of gastrointestinal symptoms, including diarrhoea, nausea, and abdominal pain [2]. However, the clinical and public health significance of Blastocystis sp. remains controversial as the protist is commonly found in both apparently healthy individuals and patients suffering from intestinal and extra-intestinal manifestations [3]. Members of the phylum microsporidia are obligate intracellular fungus-like parasites of worldwide distribution and have a marked high genetic diversity among mammalian and avian hosts. Of the 17 microsporidian species known to be infective to humans, zoonotic E. bieneusi is the most prevalent one, affecting the gastrointestinal tract and causing diarrhoea, mainly in immune-deficient individuals [4,5,6]. Among them, AIDS patients and children are especially susceptible to E. bieneusi infection [7].
Infections by Blastocystis sp. and E. bieneusi occur via the faecal-oral route through direct contact with infected hosts or due to consumption of food or water contaminated with cysts or spores [8,9,10], thus transmission through environmental contamination by competent animal host species is an important aspect of their epidemiology.
The genetic diversity and host specificity/range of both parasites have been the focus of intense research in recent years. At present, 27 genetically distinct small subunit ribosomal RNA (ssu) lineages or subtypes (STs) have been distinguished within Blastocystis sp. [11,12,13], and this number is likely to increase in the near future. Of them, ST1-ST4 account for ca. 90% of the human infections reported globally [14]. ST5 is commonly isolated from livestock, ST6 and ST7 from birds, and ST8 from arboreal non-human primates [15,16,17]). In addition to humans, ST9 has been recently described in poultry and non-human primates [18,19]. The fact that ST5‒ST8, ST10, ST12, and ST14 have been only sporadically found in humans has been interpreted as indicative of zoonotic activity [20,21,22]. ST11, ST13, and ST15‒ST17 have only been documented in non-human species so far [20,23]. Similarly, ST21 and ST23‒ST26 have been isolated from domestic and wild ruminants [11], ST27-ST29 in avian species [24,25], and ST30 and ST31 in wild ungulates [13].
On the other hand, DNA sequence analysis of the ribosomal internal transcribed spacer (ITS) region is widely used for the genetic characterization of E. bieneusi isolates and the assessment of infection sources, transmission, and zoonotic potential [7]. More than 500 genotypes of E. bieneusi have been described and allocated into 11 distinct genetic groups [4,26]. Groups 1 and 2 contain potentially zoonotic genotypes, whereas the remaining (Groups 3‒11) comprise host-adapted genotypes associated with specific animal species [4,7]. Humans are infected predominately by genetic variants within Group 1, which has over 300 genotypes, many of them with a loose host range. Group 2, in contrast, contains nearly 100 genotypes and was initially regarded as a ruminant-specific group [7].
In Spain, the molecular epidemiology of Blastocystis sp. and E. bieneusi is largely unknown. Few surveys have attempted to investigate the genetic diversity of these protist species in humans (including paediatric and clinical) [27,28,29]. Regarding animal hosts, Blastocystis sp. and E. bieneusi have been reported in livestock (pigs) [30], pets (dogs, cats) [31], and captive non-human primates [32] and animal populations and wildlife (leporids, carnivores) [33,34]. In contrast, only a single microscopy-based survey has investigated the presence of Blastocystis sp. in cattle [35], whereas no studies have ever attempted to identify the presence of E. bieneusi in this host in Spain. Although limited, previous studies conducted globally have demonstrated that cattle can harbour both zoonotic and enzootic Blastocystis subtypes and E. bieneusi genotypes [7,16,17,36,37,38,39,40,41]. The molecular characterization of Blastocystis sp. and E. bieneusi in Spanish cattle has not been investigated, nor has the potential role of this host as a natural reservoir of human blastocystosis or microsporidiosis by E. bieneusi been elucidated. Therefore, the aim of this cross-sectional epidemiological study was to determine the occurrence, genetic diversity, and zoonotic potential of Blastocystis sp. and E. bieneusi in cattle in the province of Álava, Northern Spain.
2. Materials and Methods
2.1. Sampling
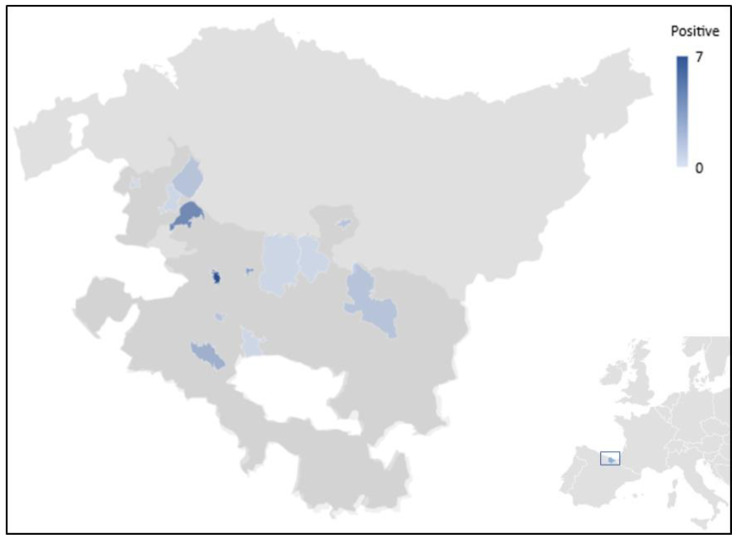
Faecal samples from asymptomatic adult dairy and beef cattle were collected from the rectum using disposable plastic bags taking advantage of routine bovine paratuberculosis control programmes in 2019 (July) and 2020 (April and August) in Álava, Northern Spain. The investigated cattle belonged to the counties of Añana, Ayala, Agurain, and Zuia (Figure 1). Collected faecal samples were transported in cooled boxes to the Livestock Laboratory of the Regional Government of Álava in Vitoria-Gasteiz, Álava (Spain) for subsequent analysis. Samples were processed within 72 h of collection.
Figure 1.
Map showing the geographical location of the sampling points included in the present study and the corresponding gradient of Blastocystis-positive results. The province of Álava (shaded in dark grey) is the Southernmost province of the Basque Autonomous Community in Northern Spain (highlighted in blue at the bottom right corner of the picture).
2.2. DNA Extraction and Purification
Total DNA was extracted from an aliquot (∼1 g) of each faecal sample using the DNA Extract-VK kit (Vacunek, Derio, Spain) according to the manufacturer’s instructions. Purified DNA samples (100 µL) were stored at −20 °C and shipped to the Parasitology Reference and Research Laboratory of the National Centre for Microbiology, Majadahonda (Spain) for further molecular analysis.
2.3. Molecular Detection of Blastocystis sp.
Identification of Blastocystis sp. was achieved by a direct PCR protocol targeting the ssu rRNA gene of the parasite [42]. The assay uses the pan-Blastocystis, barcode primer pair BhRDr (5’–GAGCTTTTTAACTGCAACAACG–3´) and RD5 (5´–ATCTGGTTGATCCTGCCAGT–3´) to amplify a PCR product of ca. 600 bp. Amplification reactions (25 μL) included 5 μL of template DNA and 0.5 μM of each primer. Cycling conditions consisted of one step of 95 °C for 3 min, followed by 30 cycles of 1 min each at 94, 59, and 72 °C, with an additional 2 min final extension at 72 °C.
2.4. Molecular Detection of Enterocytozoon bieneusi
Detection of E. bieneusi was conducted by a nested PCR protocol to amplify the internal transcribed spacer (ITS) region as well as portions of the flanking large and small subunit of the ribosomal RNA gene, as previously described [43]. The outer primer sets EBITS3 (5´–GGTCATAGGGATGAAGAG–3´) and EBTIS4 (5´–TTCGAGTTCTTTCGCGCTC–3´) and the inner primer sets EBITS1 (5´–GCTCTGAATATCTATGGCT–3´) and EBITS2.4 (5´–ATCGCCGACGGATCCAAGTG–3´) were used to generate a final PCR product of ca. 390 bp. Cycling conditions for the primary PCR consisted of one step of 94 °C for 3 min, followed by 35 cycles of amplification (denaturation at 94 °C for 30 s, annealing at 57 °C for 30 s, and elongation at 72 °C for 40 s), with a final extension at 72 °C for 10 min. Conditions for the secondary PCR were identical to the primary PCR, except that only 30 cycles were carried out with an annealing temperature of 55 °C.
All the PCR protocols described above were conducted on a 2720 Thermal Cycler (Applied Biosystems, Foster City, CA, USA). Reaction mixes always included 2.5 units of MyTAQTM DNA polymerase (Bioline GmbH, Luckenwalde, Germany) and 5× MyTAQTM Reaction Buffer containing 5 mM dNTPs and 15 mM MgCl2. Laboratory-confirmed positive and negative DNA samples for each parasitic species investigated were routinely used as controls and included in each round of PCR. PCR amplicons were visualized on 2% D5 agarose gels (Conda, Madrid, Spain) stained with Pronasafe nucleic acid staining solution (Conda). A 100 bp DNA ladder (Boehringer Mannheim GmbH, Baden-Wurttemberg, Germany) was used for the sizing of the obtained amplicons.
2.5. Sanger Sequencing and Sequence Analysis
Positive-PCR products of the expected sizes were sequenced in both directions using internal primer sets. Capillary DNA sequencing electrophoresis were used, utilizing BigDye® Terminator chemistry on an ABI PRISM 3130 automated DNA sequencer (Applied Biosystems, Foster City, CA, USA).
Raw sequencing data in both forward and reverse directions were viewed using the Chromas Lite version 2.1 (TechnelysiumPty Ltd., South Brisbane, Australia) sequence analysis program (https://technelysium.com.au/wp/chromas/, accessed on 10 September 2021). The BLAST tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 10 September 2021) was used to compare nucleotide sequences with those retrieved from the NCBI GenBank database. Generated DNA consensus sequences were aligned to appropriate reference sequences using the MEGA version 6 software [44] to identify Blastocystis STs and E. bieneusi genotypes. The E. bieneusi sequences obtained in this study have been deposited in GenBank under accession number MZ666878.
2.6. Blastocystis Subtype Identification Using Next Generation Amplicon Sequencing
DNA aliquots of potential Blastocystis-positive samples by conventional ssu-PCR were shipped to the Environmental Microbial and Food Safety Laboratory, Agricultural Research Service, Beltsville, MD, (USA). A next generation amplicon sequencing strategy was used to assess intra-isolate Blastocystis molecular diversity as previously described [45]. Briefly, a PCR using primers ILMN_Blast505_532F (5′–TCGTCGGCAGCGTCAGATGTGTATAAGAGACAGGGAGGTAGTGACAATAAATC–3′) and ILMN_Blast998_1017R (5′–GTCTCGTGGGCTCGGAGATGTGTATAAGAGACAGTGCTTTCGCACTTGTTCATC–3′) was used to screen all positive/suspected samples by conventional PCR. These primers amplify a fragment of the ssu rRNA gene (ca. 500 bp) and are identical to Blast505_532F/Blast998_1017R [46], with the exception that they contain the Illumina overhang adapter sequences (underlined) on the 5′ end. Final libraries were quantified by A Qubit (Invitrogen, Carlsbad, CA, USA) was used to fluorometrically quantify the sequencing libraries in order to normalize their concentration. Libraries were then pooled and diluted again to an 8 pM final loading concentration and spiked with 20% PhiX control. Sequencing was performed using an Illumina MiSeq using 600 cycle, v3 chemistry (Illumina, San Diego, CA, USA). Read pairs generated by the MiSeq were processed using an in-house pipeline that leveraged the following open source linux tools: BBTools package v38.82 [47], VSEARCH v2.15.1 [48], and BLAST+ 2.11.0 [49]. The processing steps included the merging of read pairs, quality and length filtering, sequence denoising, and chimera filtering. Filtered reads were then clustered into operational taxonomic units (OTUs) within each sample using a 98% identity threshold. Any OTU with an abundance below 100 sequences was filtered out and the remaining OTUs were once more checked for chimeric sequences within each sample. OTUs were then aligned to Blastocystis references from NCBI using Blast+. An alignment length cut-off of 400 bp was used to filter out short blast hits. Raw FASTQ files were submitted to NCBI’s sequence read archive under project PRJNA747253 and accession numbers SRR19164320–SRR19164427. Final OTU sequences generated by this study were submitted to GenBank under the accession numbers MZ664502–MZ664552.
2.7. Statistical Analysis
The prevalence of Blastocystis sp. and E. bieneusi infections, including 95% confidence intervals (95% CI), was calculated. The chi-squared test was used to compare Blastocystis sp. and E. bieneusi infection rates by the production system (dairy or beef) or county of origin of the surveyed cattle population. A p value < 0.05 was considered evidence of statistical significance. Analyses were conducted on R software version 3.6.0 (R Foundation for Statistical Computing, Austria, Vienna).
3. Results
3.1. Detection of Blastocystis sp. and E. bieneusi by PCR
A total of 336 faecal samples from dairy (50.0%, 168/336) and beef (19.3%, 65/336) cattle were collected during the study period. The remaining faecal samples (30.7%, 103/336) were from cattle whose production system was unknown. According to the county of origin, sampled animals were from Añana (3.3%, 11/336), Ayala (31.5%, 106/336), Llanada Alavesa (0.3%, 1/336), and Zuia-Gorbialdea (35.1%, 118/336). Origin was unknown in 29.8% (100/336) of the surveyed cattle due to incomplete sample registration at sampling (Table 1).
Table 1.
PCR-based infection rates of Blastocystis sp. and Enterocytozoon bieneusi in cattle according to production system, Álava (Spain) 2019–2020.
| Blastocystis sp. | Enterocytozoon bieneusi | ||||
|---|---|---|---|---|---|
| Variable | n | Positive (n) | % | Positive (n) | % |
| Production system | |||||
| Dairy | 168 | 38 | 22.6 | 2 | 1.2 |
| Beef | 65 | 21 | 32.3 | 0 | 0.0 |
| Unknown | 103 | 49 | 47.6 | 0 | 0.0 |
| County | |||||
| Añana | 11 | 6 | 54.5 | 0 | 0.0 |
| Ayala | 106 | 20 | 18.9 | 0 | 0.0 |
| Llanada Alavesa | 1 | 1 | 100 | 0 | 0.0 |
| Zuia-Gorbeialdea | 118 | 32 | 27.1 | 2 | 1.7 |
| Unknown | 100 | 49 | 49.0 | 0 | 0.0 |
| Total | 336 | 108 | 32.1 | 2 | 0.6 |
Overall, 32.1% (108/336, 95% CI: 27.2–37.4%) of the bovine samples yielded amplicons of the expected size by ssu-PCR using the pan-Blastocystis primers BhRDr/RD5. No statistically significant differences in Blastocystis carriage/infection rates were observed between dairy and beef cattle (p = 0.127). However, cattle from the Añana county harboured significantly higher Blastocystis carriage/infection rates than their counterparts from other counties (p = 0.0226). Enterocytozoon bieneusi was identified in 0.6% (2/336, 95% CI: 0.0–1.4%) of the samples analysed (Table 1), but its distribution according to production system or county of origin was not statistically assessed due to the low number of positive samples and lack of statistical power.
3.2. Molecular Characterization of Blastocystis sp. and E. bieneusi by Sanger Sequencing
Out of the 108 bovine samples that yielded potential Blastocystis-positive results by ssu-PCR, only 34.3% (37/108) could be confirmed by Sanger sequencing. Sequence analyses revealed ST10 as the most prevalent Blastocystis ST circulating in the bovine population under investigation (51.4%, 19/37), followed by ST5 (45.9%, 17/37) and ST14 (2.7%, 1/37). Near half (44.7%, 17/38) of the subtyped samples belonged to the cattle group for which management production was unknown (Table 2). Allele calling using the Blastocystis 18S online database failed to clearly determine intra-subtype allelic diversity due to the presence of ambiguous (double peak) positions. The remaining 71 isolates corresponded to poor quality sequences associated to faint bands on agarose gels (that is, a sub-optimal amount of parasitic DNA), or unreadable sequences associated with the presence of mixed sequence traces, likely representing mixed infections.
Table 2.
Diversity and frequency of Blastocystis subtypes and Enterocytozoon bieneusi genotypes confirmed by Sanger sequencing in cattle according to production system, Álava (Spain) 2019–2020. Potentially zoonotic genetic variants are in bold.
| Blastocystis sp. | Enterocytozoon bieneusi | |||
|---|---|---|---|---|
| Variable | Isolates(n) | Subtypes (n) | Isolates(n) | Genotypes (n) |
| Production system | ||||
| Dairy | 11 | ST5 (4), ST10 (7) | 2 | BEB4 (2) |
| Beef | 9 | ST5 (4), ST10 (5) | 0 | – |
| Unknown | 17 | ST5 (9), ST10 (7), ST14 (1) | 0 | – |
| Total | 37 | 2 | ||
Nucleotide sequence analysis of the two samples that tested positive for E. bieneusi by ITS-PCR revealed the presence of the BEB4 genotype, a genetic variant belonging to the zoonotic Group 2 of the parasite. These two samples were identified in dairy cattle (Table 2).
3.3. Blastocystis Subtype Identification by Next Generation Amplicon Sequencing
All Blastocystis-positive samples (n = 108) confirmed by Sanger sequencing (n = 37) or Blastocystis-suspected (n = 71) samples by ssu-PCR (unconfirmed by Sanger sequencing due to poor quality of chromatograms) were subjected to NGS analysis to further confirm the presence of the parasite and/or to determine the diversity and frequency of subtypes. NGS analysis confirmed the presence of Blastocystis sp. in 108 samples and allowed the identification of 10 distinct Blastocystis STs including ST1, ST3, ST5, ST10, ST14, ST21, and ST23–ST26 circulating in the bovine population under investigation (Table 3). These represent seven STs more than those detected by Sanger sequencing (Table 3). The three most common Blastocystis STs observed by NGS were ST10 (98.2%, 106/108), ST25 (86.1%, 93/108), and ST26 (92.6%, 101/108). Potentially zoonotic subtypes ST1 (1.9%, 2/108), ST3 (0.9%, 1/108), and ST5 (13.0%, 14/108) were observed in the surveyed bovine population; among them, only ST5 was also detected by Sanger sequencing.
Table 3.
Diversity and frequency of Blastocystis subtypes identified by Next Generation Amplicon Sequencing in cattle, Álava (Spain) 2019–2020. Results obtained by Sanger sequencing are shown for comparative purposes. Potentially zoonotic genetic variants are in bold.
| Subtype | Next Generation Amplicon Sequencing | Sanger Sequencing |
|---|---|---|
| ST1 | 1.9% (2/108) | Not detected |
| ST3 | 0.9% (1/108) | Not detected |
| ST5 | 13.0% (14/108) | 45.9% (17/37) |
| ST10 1 | 98.2% (106/108) | 51.4% (19/37) |
| ST14 1 | 63.0% (68/108) | 2.7% (1/37) |
| ST21 | 64.8% (70/108) | Not detected |
| ST23 | 35.2% (38/108) | Not detected |
| ST24 | 25.9% (28/108) | Not detected |
| ST25 | 86.1% (93/108) | Not detected |
| ST26 | 93.5% (101/108) | Not detected |
1 Subtypes with zoonotic potential but rarely seen in humans. See reference [22].
Remarkably, NGS analyses revealed that all Blastocystis-positive cattle carried mixed infections involving 2–8 STs of the parasite in 31 different combinations (Table 4 and Table S1). The most common mixed infections were those involving ST10 + ST25 + ST26 (13.9%, 15/108), ST10 + ST14 + ST21 + ST25 + ST26 (13.9%, 15/108), and ST10 + ST14 + ST21 + ST23 + ST25 + ST26 (11.1%, 12/108).
Table 4.
Blastocystis subtype combinations observed by NGS in cattle, Álava (Spain) 2019–2020. Potentially zoonotic genetic variants are in bold.
| Number of Subtypes in Co-Infection | Subtype Combinations | Samples (n) |
|---|---|---|
| 2 | ST10 + ST25 | 1 |
| ST25 + ST26 | 1 | |
| 3 | ST5 + ST10 + ST25 | 1 |
| ST5 + ST10 + ST14 | 1 | |
| ST10 + ST14 + ST24 | 2 | |
| ST10 + ST21 + ST26 | 1 | |
| ST10 + ST25 + ST26 | 15 | |
| ST14 + ST25 + ST26 | 1 | |
| 4 | ST5 + ST10 + ST25 + ST26 | 3 |
| ST10 + ST14 + ST21 + ST24 | 1 | |
| ST10 + ST14 + ST25 + ST26 | 5 | |
| ST10 + ST21 + ST23 + ST26 | 1 | |
| ST10 + ST21 + ST24 + ST26 | 1 | |
| ST10 + ST21 + ST25 + ST26 | 4 | |
| ST10 + ST23 + ST25 + ST26 | 4 | |
| 5 | ST5 + ST10 + ST14 + ST25 + ST26 | 1 |
| ST5 + ST10 + ST14 + ST24 + ST26 | 1 | |
| ST10 + ST14 + ST21 + ST24 + ST26 | 4 | |
| ST10 + ST14 + ST21 + ST25 + ST26 | 15 | |
| ST10 + ST21 + ST23 + ST25 + ST26 | 8 | |
| ST10 + ST14 + ST24 + ST25 + ST26 | 1 | |
| 6 | ST5 + ST10 + ST14 + ST21 + ST25 + ST26 | 4 |
| ST5 + ST10 + ST14 + ST23 + ST25 + ST26 | 1 | |
| ST10 + ST14 + ST21 + ST23 + ST24 + ST25 | 1 | |
| ST10 + ST14 + ST21 + ST23 + ST24 + ST26 | 3 | |
| ST10 + ST14 + ST21 + ST23 + ST25 + ST26 | 12 | |
| ST10 + ST14 + ST21 + ST24 + ST25 + ST26 | 5 | |
| ST10 + ST21 + ST23 + ST24 + ST25 + ST26 | 1 | |
| 7 | ST1 + ST5 + ST10 + ST14 + ST21 + ST25 + ST26 | 1 |
| ST10 + ST14 + ST21 + ST23 + ST24 + ST25 + ST26 | 7 | |
| 8 | ST3 + ST10 + ST14 + ST21 + ST23 + ST24 + ST25 + ST26 | 1 |
4. Discussion
The epidemiology of the stramenopile Blastocystis sp. and the microsporidia E. bieneusi in livestock in general, and cattle in particular, is largely unexplored in Spain. In the case of cattle, previous studies only used conventional microscopy for detection, so the genetic variants of these enteric parasites circulating in bovines remains unknown. This molecular-based epidemiological study describes, for the first time in the country, the genetic diversity of Blastocystis sp. and E. bieneusi in cattle in Northern Spain.
In Spain, few studies have examined by conventional (formaline-ethyl acetate sedimentation or Faust flotation concentration and light microscopy) methods the presence of Blastocystis sp. in livestock and captive animals. These surveys were constricted by limited diagnostic sensitivity, so documented Blastocystis occurrence rates were likely underestimated. Thus, prevalence rates of 1.8% (10/554) were reported in cattle from 30 farms in Aragon (Northeaster Spain) [35], and of 7.5% (27/360) in swine from 17 farms in the same region [50]. Blastocystis sp. has also been described at a prevalence rate of 58.0% (291/502) in ostriches from 111 Spanish farms [51]. In addition, Blastocystis sp. has been found in 15.4% (67/432) of captive animals in a zoo in Southern Spain [52], in 23.6% (232/984) of birds in an ornithological garden in the same region [53], and in non-human primates (NHP) at the Barcelona Zoo at an unspecified carriage rate [54]. A higher prevalence rate of 45.1% (23/51) was reported by PCR and Sanger sequencing methods in NHP from the Córdoba Zoo Conservation Centre in Southern Spain [32].
The relatively high Blastocystis prevalence rate (32.1%) found in adult, apparently healthy cattle in the present study agrees with previous surveys suggesting that there is a positive correlation between the age of the animal and the prevalence of the parasite [41,55]. In the case of dairy cattle, this observation could potentially be due to the lower exposure of calves to the parasite, as they are fed under more hygienic conditions than heifers or adult animals. Our prevalence data is also in line with those reported globally in cattle. Indeed, a recent systematic review and meta-analysis, in which 28 papers were considered, an average Blastocystis prevalence of 24.4% was estimated in this host species [56].
Based on the genetic variability within the ssu rRNA gene, at least 27 distinct Blastocystis STs have been identified to date [11,12,13]. Recently, third-generation sequencing technologies such as the Oxford Nanopore MinION have been used for obtaining of the full-length sequences of the ssu rRNA gene required to unambiguously identify and characterize novel Blastocystis STs from difficult matrices such as faecal material [57]. In Spain, molecular characterisation studies of Blastocystis are still limited. Regarding animal hosts, Blastocystis STs have been found in faecal samples from Iberian pigs (ST1, ST3, and ST5) and wild boars (ST5) living sympatrically [30]. A high genetic variability including ST1, ST2, ST4, ST7, and ST14 was observed in different wild carnivore species in different regions of the country [58], whereas ST1, ST3, and ST8 were reported as circulating in captive NHP [32]. Most Blastocystis STs described in Spanish animal populations were potentially zoonotic, particularly ST1–ST5. In Spain, companion dogs and cats do not seem to play a significant role as suitable hosts for Blastocystis sp. [28].
In our study, we were able to identify ten different Blastocystis STs by NGS, and six of them (ST10, ST21, ST23–ST26) have not been described before in Spain. This is in sharp contrast to our results using Sanger sequencing, where only three STs (ST5, ST10, and ST14) were detected and a large proportion (65.7%, 71/108) of the samples suspected to be Blastocystis-positive generated unreadable chromatograms due to overlapping sequences. Taken together, these findings indicate that (i) mixed infections involving different Blastocystis STs are extremely common in cattle, (ii) NGS is able not only to detect underrepresented STs in the population under study, but also to effectively discriminate among the STs involved in mixed infections, and (iii) Sanger sequencing is only effective for ST determination if a single ST is the dominant amplicon in a sample.
A wide Blastocystis genetic diversity has been documented in cattle worldwide. Sixteen different subtypes (ST1–ST7, ST10, ST12, ST14, ST17, ST21, ST23–ST26) have been de-scribed (at different frequency rates) from cattle worldwide, indicating that cattle can act as a suitable reservoir for many Blastocystis genetic variants, including several with zoonotic potential [56]. Among the subtypes reported in cattle, ST10 has been demonstrated as the most prevalent subtype in this host [59]. This is also the case for the present study, where ST10 was present in nearly all (98.2%) of the cattle analysed. Other frequent STs included ST26 (93.5%), ST25 (86.1%), ST21 (64.8%), and ST14 (63.0%). Of note, both ST25 and ST26 were detected in pre-weaned dairy heifer calves in the USA at much lower frequency rates [41], suggesting that the frequency of these STs may vary geographically or by host age. Remarkably, zoonotic ST1, ST3, and ST5 were also present in the bovine population under study, indicating that several sources of infection or even transmission pathways may be involved in the epidemiology of the parasite in this Spanish region. Studies from Iran and the USA also reported ST5 as a common finding in cattle [16,41]. The zoonotic potential of this Blastocystis ST was demonstrated in surveys conducted in Australia and China, where ST5 was not only found in swine but also in their caretakers [60,61,62].
Enterocytozoon bieneusi has been described in Spain in a wide range of animal hosts including companion (cats and dogs), production (goat, swine, rabbits, and ostriches) and free-living (pigeons, foxes) animal species [31,34,63,64,65,66], as well as in immunocompromised [27,67,68] and immunocompetent [69,70,71] individuals. In addition, spores of E. bieneusi have been identified in environmental (water) samples [72]. In the present survey, E. bieneusi was identified in 0.6% of the investigated cattle. This constitutes the first report of E. bieneusi in this host species in Spain. Worldwide, there is an abundant literature on the detection of E. bieneusi in cattle, where the reported prevalence in European countries typically varied in the range of 6–15%, which is considerably higher than that reported here [7]. An overall microsporidia (mostly attributable to E. bieneusi) prevalence in cattle of 16.6% (2,216/12,175) has been recently estimated in a large meta-analysis study including data from 79 published studies from 17 countries [73]. Studies conducted in China did not find significant differences in the occurrence of E. bieneusi among beef cattle, dairy cattle, and water buffaloes [37,39], although the opposite was true in a survey conducted in Brazil, where dairy cattle (26.2%) had a higher prevalence than beef cattle (9.7%) [38]. Domestic ruminants show a diverse pattern of genotype distribution; they are infected predominantly by Group 2 genotypes, especially BEB4 (the same E. bieneusi genotype detected in the present study), BEB6, I, and J [7]. Thus, BEB4 has been identified as infecting cattle in Argentina [74], Brazil [38], China [75], Korea [76], South Africa [77], and the USA [78]. In addition to cattle, genotype BEB4 has also been reported in pigs, yaks, donkeys, and NHP [75,79,80,81]. Furthermore, two surveys have documented the presence of BEB4 circulating in humans from China and the Czech Republic, raising concerns regarding its zoonotic potential [75,82]. It is noteworthy that domestic ruminants can also harbour well-known zoonotic Group 1 genotypes, such as EbpC, D and Type IV [83,84]; therefore, it has been indicated that E. bieneusi infections in persons from Zimbabwe may result from exposure to cattle faeces [85], suggesting a potential role of ruminants in the zoonotic transmission of E. bieneusi. In our study, we have identified only two positive samples whose genotype belongs to BEB4, within Group 2. This finding may suggest a role for cattle as a potential source of human infections by E. bieneusi, although the extent of this possibility must be confirmed in future molecular epidemiological surveys tackling bovine and human populations sharing the same environment.
5. Conclusions
This is the first molecular-based study specifically aimed at investigating the frequency, diversity, and zoonotic potential of Blastocystis subtypes and E. bieneusi genotypes circulating in Spanish cattle. We have demonstrated that Blastocystis infections involving up to eight different subtypes are extremely common in cattle, including some with well-recognized zoonotic potential such as ST1, ST3, and ST5. Importantly, we have corroborated previous molecular epidemiological studies demonstrating the usefulness and practicality of NGS for detecting underrepresented STs or complex mixed infections that would be overlooked by Sanger sequencing. Enterocytozoon bieneusi was detected at low infection rates by the zoonotic BEB4 genotype of the parasite. Overall, the molecular data presented here describes an epidemiological scenario in which different sources of infection and transmission pathways are taking part in the epidemiology of Blastocystis sp. and E. bieneusi. Given the relatively limited sample size and restricted geographical location of the study, more research should be conducted in other Spanish cattle populations to corroborate and expand the findings presented here. Our results highlight the idea that cattle could play a significant role in the transmission of these parasites through contamination of ground and surface water with Blastocystis cysts and E. bieneusi spores and serve as suitable reservoirs for human and domestic animal infections.
Acknowledgments
David González-Barrio was recipient of a “Sara Borrell” postdoctoral fellowship (CD19CIII/00011) funded by the Spanish Ministry of Science, Innovation, and Universities.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/vetsci8090191/s1, Table S1: Full dataset showing main features of bovine faecal samples at the time of collection and PCR and sequencing results for Blastocystis sp. and Enterocytozoon bieneusi.
Author Contributions
Conceptualization, M.S., G.A.C., R.C.-B., D.C., and D.G.-B.; methodology, M.S., N.S.G., A.M., G.A.C., R.C.-B., D.C., and D.G.-B.; software, A.M. and P.C.K.; validation, M.S., D.C., and D.G.-B.; formal analysis, N.A., M.S., J.G.M., A.M., A.D., P.C.K., D.C., and D.G.-B.; investigation, N.A., J.G.M., A.M., S.O., A.D., P.C.K., B.B., M.H.-d.-M., and A.S.M.; resources, M.S., G.A.C., D.C., and D.G.-B.; data curation, M.S., D.C., and D.G.-B.; writing—original draft preparation, M.S., R.C.-B., D.C., and D.G.-B.; writing—review and editing, M.S., R.C.-B., D.C., and D.G.-B.; visualization, G.A.C. and D.C.; supervision, M.S., R.C.-B., D.C., and D.G.-B.; project administration, M.S. and D.C.; funding acquisition, M.S. and D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Health Institute Carlos III (ISCIII), Ministry of Science, Innovation and Universities (Spain), grant numbers PI16CIII/00024 and USDA-ARS Project No: 8042–32000-112–00-D.
Institutional Review Board Statement
This study was carried out in accordance with Spanish legislation guidelines (RD 8/2003) and with the International Guiding Principles for Biomedical Research Involving Animals issued by the Council for International Organization of Medical Sciences and the International Council for Laboratory Animal Science (RD 53/2013). This study did not require ethics approval from an ethics committee because all the sampling and diagnostic procedures used did not affect the welfare of the animals tested. Sampling was conducted taking advantage of ongoing veterinary control campaigns to minimize animal distress.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the article and its supplementary materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Stensvold C.R. Thinking Blastocystis out of the box. Trends Parasitol. 2012;28:305. doi: 10.1016/j.pt.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 2.Ajjampur S.S., Tan K.S. Pathogenic mechanisms in Blastocystis spp. Interpreting results from in vitro and in vivo studies. Parasitol. Int. 2016;65:772–779. doi: 10.1016/j.parint.2016.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Scanlan P.D., Stensvold C.R., Rajilić-Stojanović M., Heilig H.G., De Vos W.M., O’Toole P.W., Cotter P.D. The microbial eukaryote Blastocystis is a prevalent and diverse member of the healthy human gut microbiota. FEMS Microbiol. Ecol. 2014;90:326–330. doi: 10.1111/1574-6941.12396. [DOI] [PubMed] [Google Scholar]
- 4.Li W., Feng Y., Santin M. Host specificity of Enterocytozoon bieneusi and public health implications. Trends Parasitol. 2019;35:436–451. doi: 10.1016/j.pt.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 5.Matos O., Lobo M.L., Xiao L. Epidemiology of Enterocytozoon bieneusi infection in humans. J. Parasitol. Res. 2012;2012:981424. doi: 10.1155/2012/981424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weiss L.M., Becnel J.J. Microsporidia: Pathogens of Opportunity: First Edition. Wiley Blackwell; Hoboken, NJ, USA: 2014. [Google Scholar]
- 7.Li W., Feng Y., Xiao L. Diagnosis and molecular typing of Enterocytozoon bieneusi: The significant role of domestic animals in transmission of human microsporidiosis. Res. Vet. Sci. 2020;133:251–261. doi: 10.1016/j.rvsc.2020.09.030. [DOI] [PubMed] [Google Scholar]
- 8.Tan K.S. New insights on classification, identification, and clinical relevance of Blastocystis spp. Clin. Microbiol. Rev. 2008;21:639–665. doi: 10.1128/CMR.00022-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koloren Z., Gulabi B.B., Karanis P. Molecular identification of Blastocystis sp. subtypes in water samples collected from Black sea, Turkey. Acta Trop. 2018;180:58–68. doi: 10.1016/j.actatropica.2017.12.029. [DOI] [PubMed] [Google Scholar]
- 10.Li W., Xiao L. Ecological and public health significance of Enterocytozoon bieneusi. One Health. 2021;12:100209. doi: 10.1016/j.onehlt.2020.100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stensvold C.R., Clark C.G. Pre-empting Pandora’s box: Blastocystis subtypes revisited. Trends Parasitol. 2020;36:229–232. doi: 10.1016/j.pt.2019.12.009. [DOI] [PubMed] [Google Scholar]
- 12.Maloney J.G., Santin M. Mind the gap: New full-length sequences of Blastocystis subtypes generated via Oxford Nanopore Minion sequencing allow for comparisons between full-length and partial sequences of the small subunit of the ribosomal RNA gene. Microorganisms. 2021;9:997. doi: 10.3390/microorganisms9050997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maloney J.G., Jang Y., Molokin A., George N.S., Santin M. Wide genetic diversity of Blastocystis in white-tailed deer (Odocoileus virginianus) from Maryland, USA. Microorganisms. 2021;9:1343. doi: 10.3390/microorganisms9061343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alfellani M.A., Stensvold C.R., Vidal-Lapiedra A., Onuoha E.S., Fagbenro-Beyioku A.F., Clark C.G. Variable geographic distribution of Blastocystis subtypes and its potential implications. Acta Trop. 2013;126:11–18. doi: 10.1016/j.actatropica.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 15.Alfellani M.A., Jacob A.S., Perea N.O., Krecek R.C., Taner-Mulla D., Verweij J.J., Levecke B., Tannich E., Clark C.G., Stensvold C.R. Diversity and distribution of Blastocystis sp. subtypes in non-human primates. Parasitology. 2013;140:966–971. doi: 10.1017/S0031182013000255. [DOI] [PubMed] [Google Scholar]
- 16.Badparva E., Sadraee J., Kheirandish F. Genetic diversity of Blastocystis isolated from cattle in Khorramabad, Iran. Jundishapur J. Microbiol. 2015;8:e14810. doi: 10.5812/jjm.14810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ramírez J.D., Sánchez L.V., Bautista D.C., Corredor A.F., Flórez A.C., Stensvold C.R. Blastocystis subtypes detected in humans and animals from Colombia. Infect. Genet. Evol. 2014;22:223–228. doi: 10.1016/j.meegid.2013.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Noradilah S.A., Anuar T.S., Moktar N., Lee I.L., Salleh F.M., Manap S.n.A.A., Mohtar n.S.H.M., Azrul S.M., Abdullah W.O., Nordin A., et al. Molecular epidemiology of Blastocystis sp. in animals reared by the aborigines during wet and dry seasons in rural communities, Pahang, Malaysia. Southeast Asian J. Trop. Med. Public Health. 2017;48:1151–1160. doi: 10.1186/s13071-017-2294-2. [DOI] [Google Scholar]
- 19.Ma L., Qiao H., Wang H., Li S., Zhai P., Huang J., Guo Y. Molecular prevalence and subtypes of Blastocystis sp. in primates in northern China. Transbound. Emerg. Dis. 2020;67:2789–2796. doi: 10.1111/tbed.13644. [DOI] [PubMed] [Google Scholar]
- 20.Clark C.G., van der Giezen M., Alfellani M.A., Stensvold C.R. Recent developments in Blastocystis research. Adv. Parasitol. 2013;82:1–32. doi: 10.1016/B978-0-12-407706-5.00001-0. [DOI] [PubMed] [Google Scholar]
- 21.Ramírez J.D., Sánchez A., Hernández C., Flórez C., Bernal M.C., Giraldo J.C., Reyes P., López M.C., García L., Cooper P.J., et al. Geographic distribution of human Blastocystis subtypes in South America. Infect. Genet. Evol. 2016;41:32–35. doi: 10.1016/j.meegid.2016.03.017. [DOI] [PubMed] [Google Scholar]
- 22.Khaled S., Gantois N., Ly A.T., Senghor S., Even G., Dautel E., Dejager R., Sawant M., Baydoun M., Benamrouz-Vanneste S., et al. Prevalence and subtype distribution of Blastocystis sp. in Senegalese school children. Microorganisms. 2020;8:1408. doi: 10.3390/microorganisms8091408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Alfellani M.A., Taner-Mulla D., Jacob A.S., Imeede C.A., Yoshikawa H., Stensvold C.R., Clark C.G. Genetic diversity of Blastocystis in livestock and zoo animals. Protist. 2013;164:497–509. doi: 10.1016/j.protis.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 24.Maloney J.G., Molokin A., da Cunha M.J.R., Cury M.C., Santin M. Blastocystis subtype distribution in domestic and captive wild bird species from Brazil using next generation amplicon sequencing. Parasite Epidemiol. Control. 2020;9:e00138. doi: 10.1016/j.parepi.2020.e00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maloney J.G., da Cunha M.J.R., Molokin A., Cury M.C., Santin M. Next-generation sequencing reveals wide genetic diversity of Blastocystis subtypes in chickens including potentially zoonotic subtypes. Parasitol. Res. 2021;120:2219–2231. doi: 10.1007/s00436-021-07170-3. [DOI] [PubMed] [Google Scholar]
- 26.Santin M. Enterocytozoon bieneusi. In: Xiao L., Ryan U., Feng Y., editors. Biology of Foodborne Parasites. CRC Press; Boca Raton, FL, USA: 2015. pp. 149–174. [Google Scholar]
- 27.Galván A.L., Sánchez A.M., Valentín M.A., Henriques-Gil N., Izquierdo F., Fenoy S., del Aguila C. First cases of microsporidiosis in transplant recipients in Spain and review of the literature. J. Clin. Microbiol. 2011;49:1301–1306. doi: 10.1128/JCM.01833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulos S., Köster P.C., de Lucio A., Hernández-de-Mingo M., Cardona G.A., Fernández-Crespo J.C., Stensvold C.R., Carmena D. Occurrence and subtype distribution of Blastocystis sp. in humans, dogs and cats sharing household in northern Spain and assessment of zoonotic transmission risk. Zoonoses Public Health. 2018;65:993–1002. doi: 10.1111/zph.12522. [DOI] [PubMed] [Google Scholar]
- 29.Muadica A.S., Köster P.C., Dashti A., Bailo B., Hernández-de-Mingo M., Reh L., Balasegaram S., Verlander N.Q., Ruiz Chércoles E., Carmena D. Molecular diversity of Giardia duodenalis, Cryptosporidium spp. and Blastocystis sp. in asymptomatic school children in Leganés, Madrid (Spain) Microorganisms. 2020;8:466. doi: 10.3390/microorganisms8040466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rivero-Juarez A., Dashti A., López-López P., Muadica A.S., Risalde M.L.A., Köster P.C., Machuca I., Bailo B., de Mingo M.H., Dacal E., et al. Protist enteroparasites in wild boar (Sus scrofa ferus) and black Iberian pig (Sus scrofa domesticus) in southern Spain: A protective effect on hepatitis E acquisition? Parasit. Vectors. 2020;13:281. doi: 10.1186/s13071-020-04152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dashti A., Santín M., Cano L., de Lucio A., Bailo B., de Mingo M.H., Köster P.C., Fernández-Basterra J.A., Aramburu-Aguirre J., López-Molina N., et al. Occurrence and genetic diversity of Enterocytozoon bieneusi (Microsporidia) in owned and sheltered dogs and cats in Northern Spain. Parasitol. Res. 2019;118:2979–2987. doi: 10.1007/s00436-019-06428-1. [DOI] [PubMed] [Google Scholar]
- 32.Köster P.C., Dashti A., Bailo B., Muadica A.S., Maloney J.G., Santín M., Chicharro C., Migueláñez S., Nieto F.J., Cano-Terriza D., et al. Occurrence and genetic diversity of protist parasites in captive non-human primates, zookeepers, and free-living sympatric rats in the Córdoba Zoo Conservation Centre, Southern Spain. Animals. 2021;11:700. doi: 10.3390/ani11030700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martínez-Padilla A., Caballero-Gómez J., Magnet Á., Gómez-Guillamón F., Izquierdo F., Camacho-Sillero L., Jiménez-Ruiz S., Del Águila C., García-Bocanegra I. Zoonotic Microsporidia in wild lagomorphs in Southern Spain. Animals. 2020;10:2218. doi: 10.3390/ani10122218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Santín M., Calero-Bernal R., Carmena D., Mateo M., Balseiro A., Barral M., Lima Barbero J.F., Habela M.Á. Molecular characterization of Enterocytozoon bieneusi in wild carnivores in Spain. J. Eukaryot. Microbiol. 2018;65:468–474. doi: 10.1111/jeu.12492. [DOI] [PubMed] [Google Scholar]
- 35.Quílez J., Sánchez-Acedo C., Clavel A., Causapé A.C. Occurrence of Blastocystis sp. in cattle in Aragón, northeastern Spain. Parasitol. Res. 1995;81:703–705. doi: 10.1007/BF00931851. [DOI] [PubMed] [Google Scholar]
- 36.Stensvold C.R., Alfellani M.A., Nørskov-Lauritsen S., Prip K., Victory E.L., Maddox C., Nielsen H.V., Clark C.G. Subtype distribution of Blastocystis isolates from synanthropic and zoo animals and identification of a new subtype. Int. J. Parasitol. 2009;39:473–479. doi: 10.1016/j.ijpara.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Ma J., Li P., Zhao X., Xu H., Wu W., Wang Y., Guo Y., Wang L., Feng Y., Xiao L. Occurrence and molecular characterization of Cryptosporidium spp. and Enterocytozoon bieneusi in dairy cattle, beef cattle and water buffaloes in China. Vet. Parasitol. 2015;207:220–227. doi: 10.1016/j.vetpar.2014.10.011. [DOI] [PubMed] [Google Scholar]
- 38.da Silva Fiuza V.R., Lopes C.W., de Oliveira F.C., Fayer R., Santin M. New findings of Enterocytozoon bieneusi in beef and dairy cattle in Brazil. Vet. Parasitol. 2016;216:46–51. doi: 10.1016/j.vetpar.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 39.Wang X.T., Wang R.J., Ren G.J., Yu Z.Q., Zhang L.X., Zhang S.Y., Lu H., Peng X.Q., Zhao G.H. Multilocus genotyping of Giardia duodenalis and Enterocytozoon bieneusi in dairy and native beef (Qinchuan) calves in Shaanxi province, northwestern China. Parasitol. Res. 2016;115:1355–1361. doi: 10.1007/s00436-016-4908-6. [DOI] [PubMed] [Google Scholar]
- 40.Tang C., Cai M., Wang L., Guo Y., Li N., Feng Y., Xiao L. Genetic diversity within dominant Enterocytozoon bieneusi genotypes in pre-weaned calves. Parasit. Vectors. 2018;11:170. doi: 10.1186/s13071-018-2768-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maloney J.G., Lombard J.E., Urie N.J., Shivley C.B., Santin M. Zoonotic and genetically diverse subtypes of Blastocystis in US pre-weaned dairy heifer calves. Parasitol. Res. 2019;118:575–582. doi: 10.1007/s00436-018-6149-3. [DOI] [PubMed] [Google Scholar]
- 42.Scicluna S.M., Tawari B., Clark C.G. DNA barcoding of Blastocystis. Protist. 2006;157:77–85. doi: 10.1016/j.protis.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 43.Buckholt M.A., Lee J.H., Tzipori S. Prevalence of Enterocytozoon bieneusi in swine: An 18-month survey at a slaughterhouse in Massachusetts. Appl. Environ. Microbiol. 2002;68:2595–2599. doi: 10.1128/AEM.68.5.2595-2599.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tamura K., Stecher G., Peterson D., Filipski A., Kumar S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maloney J.G., Molokin A., Santin M. Next generation amplicon sequencing improves detection of Blastocystis mixed subtype infections. Infect. Genet. Evol. 2019;73:119–125. doi: 10.1016/j.meegid.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 46.Santín M., Gómez-Muñoz M.T., Solano-Aguilar G., Fayer R. Development of a new PCR protocol to detect and subtype Blastocystis spp. from humans and animals. Parasitol. Res. 2011;109:205–212. doi: 10.1007/s00436-010-2244-9. [DOI] [PubMed] [Google Scholar]
- 47.Bushnell B. BBMap Download. SourceForge.net. 2014. [(accessed on 27 April 2020)]. Available online: https://sourceforge.net/projects/bbmap/
- 48.Rognes T., Flouri T., Nichols B., Quince C., Mahé F. VSEARCH: A versatile open source tool for metagenomics. PEER J. 2016;4:e2584. doi: 10.7717/peerj.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Camacho C., Coulouris G., Avagyan V., Ma N., Papadopoulos J., Bealer K., Madden T.L. BLAST+: Architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Quilez J., Clavel A., Sanchez-Acedo C., Causape A.C. Detection of Blastocystis sp. in pigs in Aragon (Spain) Vet. Parasitol. 1995;56:345–348. doi: 10.1016/0304-4017(94)00682-3. [DOI] [PubMed] [Google Scholar]
- 51.Ponce Gordo F., Herrera S., Castro A.T., García Durán B., Martínez Díaz R.A. Parasites from farmed ostriches (Struthio camelus) and rheas (Rhea americana) in Europe. Vet. Parasitol. 2002;107:137–160. doi: 10.1016/S0304-4017(02)00104-8. [DOI] [PubMed] [Google Scholar]
- 52.Pérez Cordón G., Hitos Prados A., Romero D., Sánchez Moreno M., Pontes A., Osuna A., Rosales M.J. Intestinal parasitism in the animals of the zoological garden “Peña Escrita” (Almuñecar, Spain) Vet. Parasitol. 2008;156:302–309. doi: 10.1016/j.vetpar.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 53.Cordón G.P., Prados A.H., Romero D., Moreno M.S., Pontes A., Osuna A., Rosales M.J. Intestinal and haematic parasitism in the birds of the Almuñecar (Granada, Spain) ornithological garden. Vet. Parasitol. 2009;165:361–366. doi: 10.1016/j.vetpar.2009.07.027. [DOI] [PubMed] [Google Scholar]
- 54.Soledad Gómez M., Gracenea M., Montoliu I., Feliu C., Monleon A., Fernandez J., Enseñat C. Intestinal parasitism--protozoa and helminths--in primates at the Barcelona Zoo. J. Med. Primatol. 1996;25:419–423. doi: 10.1111/j.1600-0684.1996.tb00038.x. [DOI] [PubMed] [Google Scholar]
- 55.Zhu W., Tao W., Gong B., Yang H., Li Y., Song M., Lu Y., Li W. First report of Blastocystis infections in cattle in China. Vet. Parasitol. 2017;246:38–42. doi: 10.1016/j.vetpar.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 56.Shams M., Shamsi L., Sadrebazzaz A., Asghari A., Badali R., Omidian M., Hassanipour S. A systematic review and meta-analysis on the global prevalence and subtypes distribution of Blastocystis sp. infection in cattle: A zoonotic concern. Comp. Immunol. Microbiol. Infect. Dis. 2021;76:101650. doi: 10.1016/j.cimid.2021.101650. [DOI] [PubMed] [Google Scholar]
- 57.Maloney J.G., Molokin A., Santin M. Use of Oxford Nanopore MinION to generate full-length sequences of the Blastocystis small subunit (SSU) rRNA gene. Parasit. Vectors. 2020;13:595. doi: 10.1186/s13071-020-04484-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calero-Bernal R., Santín M., Maloney J.G., Martín-Pérez M., Habela M.A., Fernández-García J.L., Figueiredo A., Nájera F., Palacios M.J., Mateo M., et al. Blastocystis sp. subtype diversity in wild carnivore species from Spain. J. Eukaryot. Microbiol. 2020;67:273–278. doi: 10.1111/jeu.12772. [DOI] [PubMed] [Google Scholar]
- 59.Hublin J.S.Y., Maloney J.G., Santin M. Blastocystis in domesticated and wild mammals and birds. Res. Vet. Sci. 2021;135:260–282. doi: 10.1016/j.rvsc.2020.09.031. [DOI] [PubMed] [Google Scholar]
- 60.Yan Y., Su S., Ye J., Lai X., Lai R., Liao H., Chen G., Zhang R., Hou Z., Luo X. Blastocystis sp. subtype 5: A possibly zoonotic genotype. Parasitol. Res. 2007;101:1527–1532. doi: 10.1007/s00436-007-0672-y. [DOI] [PubMed] [Google Scholar]
- 61.Li L.H., Zhou X.N., Du Z.W., Wang X.Z., Wang L.B., Jiang J.Y., Yoshikawa H., Steinmann P., Utzinger J., Wu Z., et al. Molecular epidemiology of human Blastocystis in a village in Yunnan province, China. Parasitol. Int. 2007;56:281–286. doi: 10.1016/j.parint.2007.06.001. [DOI] [PubMed] [Google Scholar]
- 62.Wang W., Owen H., Traub R.J., Cuttell L., Inpankaew T., Bielefeldt-Ohmann H. Molecular epidemiology of Blastocystis in pigs and their in-contact humans in Southeast Queensland, Australia, and Cambodia. Vet. Parasitol. 2014;203:264–269. doi: 10.1016/j.vetpar.2014.04.006. [DOI] [PubMed] [Google Scholar]
- 63.del Aguila C., Izquierdo F., Navajas R., Pieniazek N.J., Miró G., Alonso A.I., Da Silva A.J., Fenoy S. Enterocytozoon bieneusi in animals: Rabbits and dogs as new hosts. J. Eukaryot. Microbiol. 1999;46:8S–9S. [PubMed] [Google Scholar]
- 64.Galván-Díaz A.L., Magnet A., Fenoy S., Henriques-Gil N., Haro M., Ponce-Gordo F., Miró G., Del Águila C., Izquierdo F. Microsporidia Detection and Genotyping Study of Human Pathogenic E. bieneusi in Animals from Spain. PLoS ONE. 2014;9:e92289. doi: 10.1371/journal.pone.0092289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haro M., Henriques-Gil N., Fenoy S., Izquierdo F., Alonso F., Del Aguila C. Detection and genotyping of Enterocytozoon bieneusi in pigeons. J. Eukaryot. Microbiol. 2006;53:S58–S60. doi: 10.1111/j.1550-7408.2006.00173.x. [DOI] [PubMed] [Google Scholar]
- 66.Lores B., del Aguila C., Arias C. Enterocytozoon bieneusi (microsporidia) in faecal samples from domestic animals from Galicia, Spain. Mem. Inst. Oswaldo Cruz. 2002;97:941–945. doi: 10.1590/S0074-02762002000700003. [DOI] [PubMed] [Google Scholar]
- 67.del Aguila C., Navajas R., Gurbindo D., Ramos J.T., Mellado M.J., Fenoy S., Muñoz Fernandez M.A., Subirats M., Ruiz J., Pieniazek N.J. Microsporidiosis in HIV-positive children in Madrid (Spain) J. Eukaryot. Microbiol. 1997;44:84S–85S. doi: 10.1111/j.1550-7408.1997.tb05798.x. [DOI] [PubMed] [Google Scholar]
- 68.del Aguila C., Soriano V., Navajas R., Subirats M., Fenoy S., Valencia E., Baquero M., Pieniazek N.J. Species identification of intestinal microsporidiosis in HIV-positive patients using the polymerase chain reaction. Enferm. Infecc. Microbiol. Clin. 1997;15:456–461. [PubMed] [Google Scholar]
- 69.López-Vélez R., Turrientes M.C., Garrón C., Montilla P., Navajas R., Fenoy S., del Aguila C. Microsporidiosis in travelers with diarrhea from the tropics. J. Travel Med. 1999;6:223–227. doi: 10.1111/j.1708-8305.1999.tb00522.x. [DOI] [PubMed] [Google Scholar]
- 70.Lores B., López-Miragaya I., Arias C., Fenoy S., Torres J., del Aguila C. Intestinal microsporidiosis due to Enterocytozoon bieneusi in elderly human immunodeficiency virus--negative patients from Vigo, Spain. Clin. Infect. Dis. 2002;34:918–921. doi: 10.1086/339205. [DOI] [PubMed] [Google Scholar]
- 71.Abreu-Acosta N., Lorenzo-Morales J., Leal-Guio Y., Coronado-Alvarez N., Foronda P., Alcoba-Florez J., Izquierdo F., Batista-Díaz N., Del Aguila C., Valladares B. Enterocytozoon bieneusi (microsporidia) in clinical samples from immunocompetent individuals in Tenerife, Canary Islands, Spain. Trans. R. Soc. Trop. Med. Hyg. 2005;99:855.:848. doi: 10.1016/j.trstmh.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 72.Galván A.L., Magnet A., Izquierdo F., Fenoy S., Rueda C., Fernández Vadillo C., Henriques-Gil N., del Aguila C. Molecular characterization of human-pathogenic microsporidia and Cyclospora cayetanensis isolated from various water sources in Spain: A year-long longitudinal study. Appl. Environ. Microbiol. 2013;79:449–459. doi: 10.1128/AEM.02737-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ruan Y., Xu X., He Q., Li L., Guo J., Bao J., Pan G., Li T., Zhou Z. The largest meta-analysis on the global prevalence of microsporidia in mammals, avian and water provides insights into the epidemic features of these ubiquitous pathogens. Parasit. Vectors. 2021;14:186. doi: 10.1186/s13071-021-04700-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Del Coco V.F., Córdoba M.A., Bilbao G., de Almeida Castro P., Basualdo J.A., Santín M. First report of Enterocytozoon bieneusi from dairy cattle in Argentina. Vet. Parasitol. 2014;199:112–115. doi: 10.1016/j.vetpar.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 75.Zhang X., Wang Z., Su Y., Liang X., Sun X., Peng S., Lu H., Jiang N., Yin J., Xiang M., et al. Identification and genotyping of Enterocytozoon bieneusi in China. J. Clin. Microbiol. 2011;49:2006–2008. doi: 10.1128/JCM.00372-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hwang S., Shin S.U., Kim S., Ryu J.H., Choi K.S. Zoonotic potential of Enterocytozoon bieneusi in pre-weaned Korean native calves. Parasit. Vectors. 2020;13:300. doi: 10.1186/s13071-020-04175-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abu Samra N., Thompson P.N., Jori F., Zhang H., Xiao L. Enterocytozoon bieneusi at the wildlife/livestock interface of the Kruger National Park, South Africa. Vet. Parasitol. 2012;190:587–590. doi: 10.1016/j.vetpar.2012.06.031. [DOI] [PubMed] [Google Scholar]
- 78.Santín M., Fayer R. A longitudinal study of Enterocytozoon bieneusi in dairy cattle. Parasitol. Res. 2009;105:141–144. doi: 10.1007/s00436-009-1374-4. [DOI] [PubMed] [Google Scholar]
- 79.Ma J., Cai J., Ma J., Feng Y., Xiao L. Enterocytozoon bieneusi genotypes in yaks (Bos grunniens) and their public health potential. J. Eukaryot. Microbiol. 2015;62:21–25. doi: 10.1111/jeu.12141. [DOI] [PubMed] [Google Scholar]
- 80.Zhao A., Zhang Y., Wang W., Jing B., Xing J., Tao D., Zhao W., Qi M. Enterocytozoon bieneusi in donkeys from Xinjiang, China: Prevalence, molecular characterization and the assessment of zoonotic risk. BMC Vet. Res. 2020;16:196. doi: 10.1186/s12917-020-02409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Karim M.R., Dong H., Li T., Yu F., Li D., Zhang L., Li J., Wang R., Li S., Li X., et al. Predomination and new genotypes of Enterocytozoon bieneusi in captive nonhuman primates in zoos in China: High genetic diversity and zoonotic significance. PLoS ONE. 2015;10:e0117991. doi: 10.1371/journal.pone.0117991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sak B., Brady D., Pelikánová M., Květoňová D., Rost M., Kostka M., Tolarová V., Hůzová Z., Kváč M. Unapparent microsporidial infection among immunocompetent humans in the Czech Republic. J. Clin. Microbiol. 2011;49:1064–1070. doi: 10.1128/JCM.01147-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang H.Y., Qi M., Sun M.F., Li D.F., Wang R.J., Zhang S.M., Zhao J.F., Li J.Q., Cui Z.H., Chen Y.C., et al. Prevalence and population genetics analysis of Enterocytozoon bieneusi in dairy cattle in China. Front. Microbiol. 2019;10:1399. doi: 10.3389/fmicb.2019.01399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang R., Li N., Jiang W., Guo Y., Wang X., Jin Y., Feng Y., Xiao L. Infection patterns, clinical significance, and genetic characteristics of Enterocytozoon bieneusi and Giardia duodenalis in dairy cattle in Jiangsu, China. Parasitol. Res. 2019;118:3053–3060. doi: 10.1007/s00436-019-06426-3. [DOI] [PubMed] [Google Scholar]
- 85.Gumbo T., Sarbah S., Gangaidzo I.T., Ortega Y., Sterling C.R., Carville A., Tzipori S., Wiest P.M. Intestinal parasites in patients with diarrhea and human immunodeficiency virus infection in Zimbabwe. AIDS. 1999;13:819–821. doi: 10.1097/00002030-199905070-00011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the article and its supplementary materials.