Figure 7.
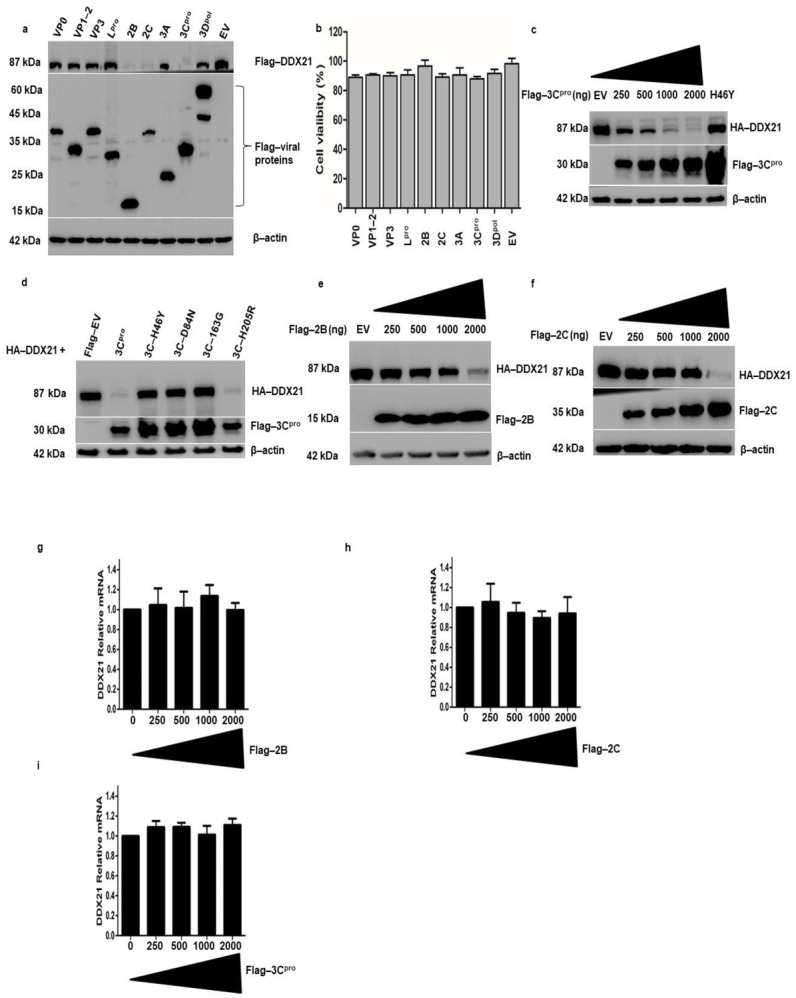
DDX21 is degraded by FMDV 2B, 2C, and 3Cpro. (a) PK-15 cells in six-well plates were co-transfected with Flag-DDX21 and VP0, VP1-2, VP3, Lpro, 2B, 2C, 3A, 3Cpro, 3Dpol, or Flag-EV. Cells were harvested after 24 h and 1× SDS loading buffer was added. The samples were analyzed by Western blot. (b) After the cells were grown to 80% confluence in 96 well plates, they were transfected with Flag-VP0, VP1-2, VP3, Lpro, 2B, 2C, 3A, 3Cpro, and 3Dpol or an empty vector for 24 h. For the MTS assay, 10 μL of CellTiter 96® AQueous One Solution Cell Proliferation Assay reagent (Promega, WI, USA) was directly added to the cells, which were then incubated for 4 h. The absorbance at 490 nm was recorded. (c) PK-15 cells were co-transfected with HA-DDX21 (2 µg) and Flag-3Cpro (250, 500, 1000, or 2000 ng) or Flag-EV (2 µg). Cell lysates were collected in 1× SDS loading buffer and analyzed by Western blotting. (d) PK-15 cells on six-well plates were co-transfected with HA-DDX21 (2 µg) and Flag-3Cpro, H46Y, D84N, 163G, H205R, or Flag-EV (2 µg). Samples were collected at 24 h post-transfection and analyzed by Western blotting. (e) PK-15 cells on six-well plates were co-transfected with HA-DDX21 (2 µg) and Flag-2B (250, 500, 1000, or 2000 ng) or Flag-EV (2 µg). Cell lysates were collected 24 h post-transfection in 1× SDS loading buffer and analyzed by Western blotting. (f) PK-15 cells were co-transfected with HA-DDX21 (2 µg) and Flag-3Cpro (250, 500, 1000, or 2000 ng) or Flag-EV (2 µg). Cell lysates were collected 24 h post-transfection in 1× SDS loading buffer and analyzed by Western blotting. (g–i) PK-15 cells were cultured on a six-well plate. At 80% confluence, cells were transfected with an increasing concentration of Flag-2B, Flag-2C, and Flag-3Cpro (0, 250, 500, 1000, and 2000 ng). Twenty-four hours post transfection; RNA was extracted and the level of DDX21 mRNA was determined by qRT-PCR.
