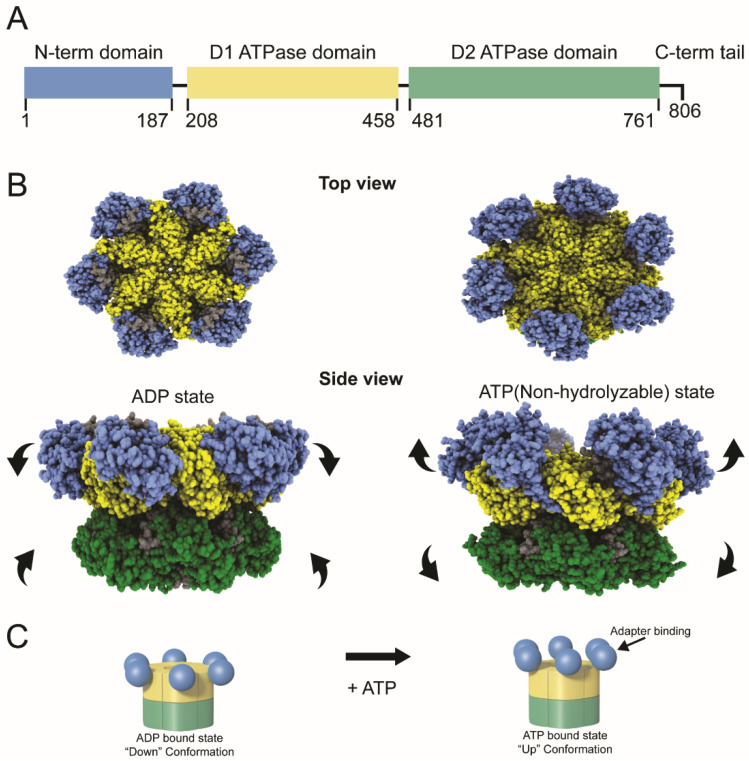
Figure 1.
Domain structure and conformational changes in the VCP/p97 ATPase. (A) Diagram of the domains within the VCP/p97 monomer. Amino acid positions of each domain are indicated. (B) Cryoelectron microscopic images of VCP hexamers in the presence of ADP and ATP (non-hydrolyzable analog). The top view shows the propeller-like appearance of the six monomer subunits surrounding a central channel. The side view depicts the conformational changes of each monomer in the presence of ADP or ATPγS. The N-terminus and D1 and D2 domains are depicted in blue, yellow, and green respectively; other regions are shown in gray (PDB:5ftl and PDB:5ftn). [3]. In living cells, the unfolding ability of VCP is likely to be dependent on the sequential conformational changes in adjacent monomers to pull the substrate through the central pore (C) Cartoon depiction of the conformational switch in the N-terminal domains within the p97 hexamer. Color coding of the domains corresponds to panel A. In the ADP-bound state, the N-terminal domains primarily are in the “down” conformation. In the ATP-bound state, the N-terminal domains are in the “up” conformation for interactions with other proteins, often with adapters that recognize polyubiquitylated proteins. It is likely that many VCP hexamers have a mixture of “up” and “down” conformations within monomers, which allows the hand-over-hand unfolding and extraction mechanism.