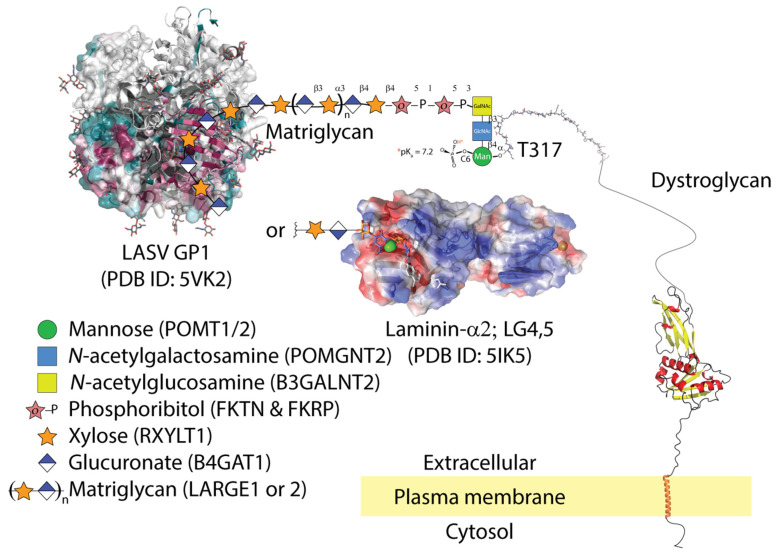
Figure 1.
LASV GP1 is able to bind matriglycan (xylose and glucuronate), but only gains entry to cells that co-express apoptotic phagocytic machinery. The molecular details of LASV GP1 binding to matriglycan are unknown. Matriglycan is polymerized on a primer of extended phosphocore M3 on threonine-317 and possibly 379 of α-dystroglycan. The core M3 trisaccharide is phosphorylated by Protein O-Mannosyl Kinase (POMK); other glycosyltransferases are listed in parentheses next to their corresponding sugars. The conserved surface residues of LASV trimer from 5VK2 are shown as a gradient of magenta (conserved) to green (non-conserved); (accessed on 6 July 2021: https://consurf.tau.ac.il/). LASV GP1 binding displaces LG domains from matriglycan. The semi-transparent electrostatic surface of LG4-5 domains from laminin-2α is shown binding a unit of xylose-glucuronate via calcium (green sphere). Parts of the dystroglycan structure were downloaded from AlphaFold [26].