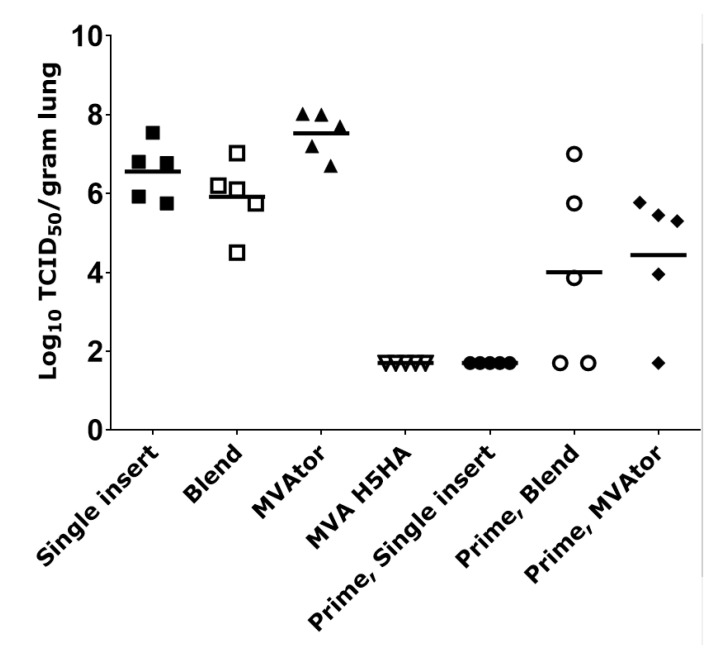
Figure 5.
Lung viral burdens after immunization and H5N1 challenge. Mice were immunized two times using a single MVA vaccine that encoded M1 + NP + METRC (“single insert”) or a combination of three separate MVA vaccines each encoding M1 or NP or METRC (“Blend”). “Prime” indicates previous mild infection with PR8 H1N1 INFV A, 21 days prior to vaccine administration. A TCID50 assay was performed to quantify infectious H5N1 virus in lungs (N = 5 per group) 4 days after A/VN/1203/04 challenge (H5N1). The horizontal bar for each group represents the arithmetic mean Log10 TCID50 per gram tissue. Individual dots represent the results of each animal. The limit of detection for this assay was 1.7 Log10 TCID50. Lung viral burden on day 2 (data not shown) had nearly identical results compared to day 4. Without PR8 priming, only the MVA H5HA vaccine was different (p < 0.0001, ANOVA followed by Tukey–Kramer test) from the MVAtor control. If delivered after PR8 priming, MVA encoding M1 + NP + METRC as a single insert again was significantly different from MVAtor with priming (p = 0.019). Priming gave protective advantage to all test vaccines including the MVAtor negative control. Limit of detection was log 1.7.