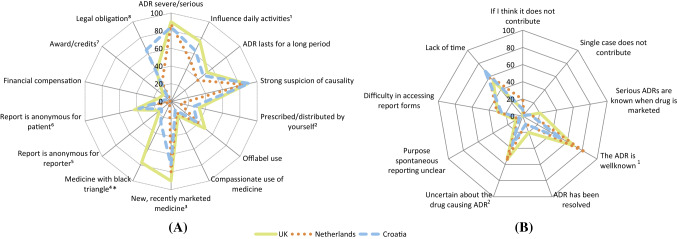
Fig. 1.
Healthcare professionals’ responses to the question a ‘What would motivate you to report an adverse drug reaction?’ (3 missing; 1 ‘nothing would motivate me’; 11 other answers), and b ‘What are your reasons for not reporting an ADR?’ (48 ‘none’; 9 other answers). *A black triangle is assigned to medicinal products that are subjected to additional safety monitoring [34]. ADR adverse drug reaction. Fig. 1A: 1Overall P = 0.023. V = 0.16. The Netherlands and the UK significantly different (P = 0.006). 2Overall P = 0.019. V = 0.16. The Netherlands significantly different the UK (P = 0.005). 3Overall P = 0.013. V = 0.17. Croatia and the UK significantly different (P = 0.004). 4Overall P < 0.001. V = 0.52. P < 0.001 for each specific comparison between two countries. 5Overall P = 0.031. V = 0.15. The Netherlands significantly different from Croatia (P = 0.014) and the UK (P = 0.007). 6Overall P = 0.005. V = 0.19. The Netherlands significantly different from Croatia (P = 0.003) and the UK (P = 0.002). 7Overall P = 0.006. V = 0.18. The UK and the Netherlands significantly different (P = 0.010). 8Overall P < 0.001. V = 0.34. Croatia significantly different from the Netherlands and the UK (P < 0.001 for both). Fig. 1B: 1Overall P < 0.001. V = 0.26. Croatia and the Netherlands significantly different (P < 0.001). 2Overall P = 0.015. V = 0.18. Croatia and the Netherlands significantly different (P = 0.015)