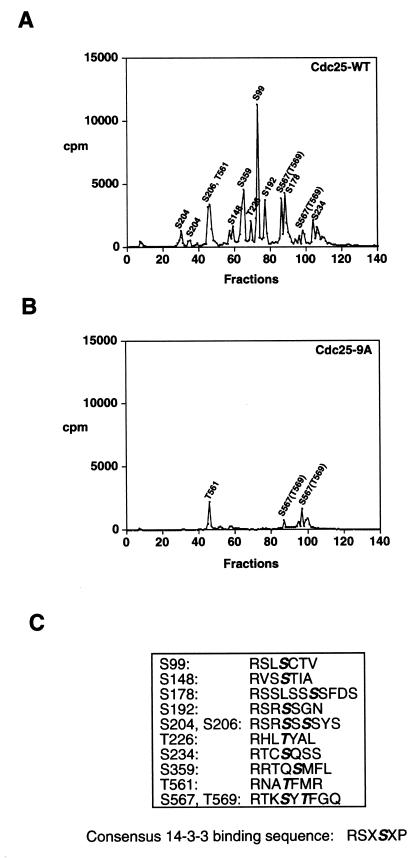
FIG. 1.
Identification of 12 residues of Cdc25 phosphorylated by Cds1 in vitro. (A and B) Cdc25-WT and Cdc25-9A were purified as GST fusion proteins, and kinase assays were performed in vitro in the presence of the Cds1 protein kinase. Radiolabeled GST-Cdc25-WT (A) and GST-Cdc25-9A (B) were digested with trypsin, and the tryptic peptides were resolved by reverse-phase HPLC. Column fractions were collected and monitored for the presence of radioactivity. Identified residues are shown above the fraction in which they elute. Peptides containing phosphorylated S234 and S567/T569 elute in more than one fraction. (C) Phosphorylation sites (bold and italics) and neighboring residues. The consensus sequence for 14-3-3 binding as defined by Muslin et al. (40) is indicated.