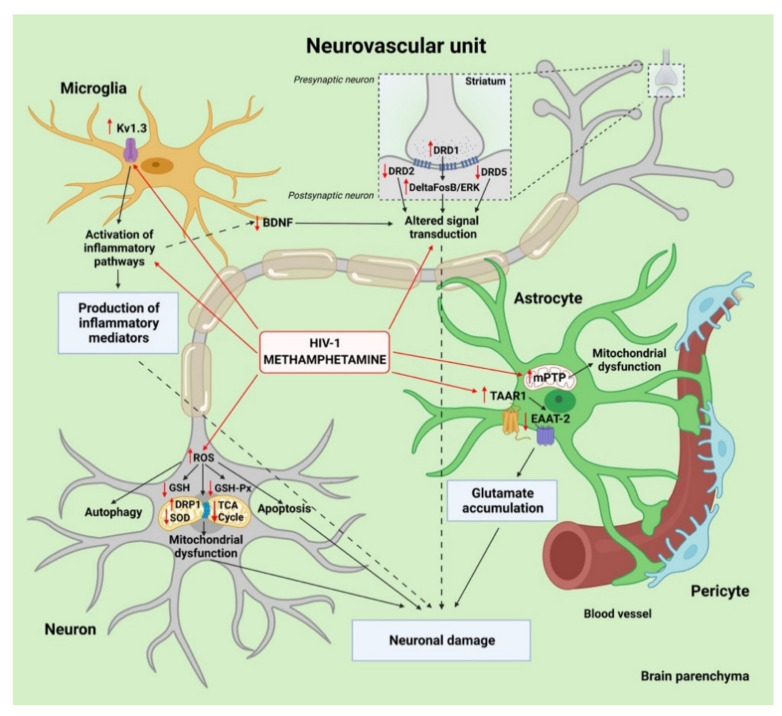
Figure 3.
Synergistic cooperation between HIV-1 and methamphetamine (METH) in the neurovascular unit (NVU) potentiates neuroinflammation and enhances neuronal damage. HIV-1 enters the brain via infected monocytes and CD4+ lymphocytes that cross the blood brain barrier (BBB). Once inside the brain, infected macrophages facilitate productive infection and release free virions into the brain parenchyma that infect neighboring microglia and, to some degree, astrocytes and pericytes. This infection leads to the release of neurotoxic factors and to enhanced activation of microglia. METH readily crosses the BBB due to its small size and significantly potentiates neuronal damage, mediated by microglia activation of voltage-gated potassium channel KV1.3. METH also downregulates the release of brain-derived neurotrophic factor (BDNF) from HIV-1-infected microglia. METH and HIV-1 cause astrocyte dysfunction by the opening of mitochondrial permeability transition pores (mPTPs) and modulating TAAR1/EAAT2 signaling pathways involved in glutamate clearance from the extracellular space. The other synergistic effects of METH and HIV-1 include mitochondrial fission mediated by dynamin-related protein 1 (DRP1) and impairment of the tricarboxylic acid (TCA) cycle in neurons. Furthermore, the combination of METH and HIV-1 induces neuronal oxidative damage by downregulating levels of glutathione (GSH), antioxidant defense enzymes glutathione peroxidase (GSH-PX), and superoxide dismutase (SOD). Created with BioRender.com.