FIG. 4.
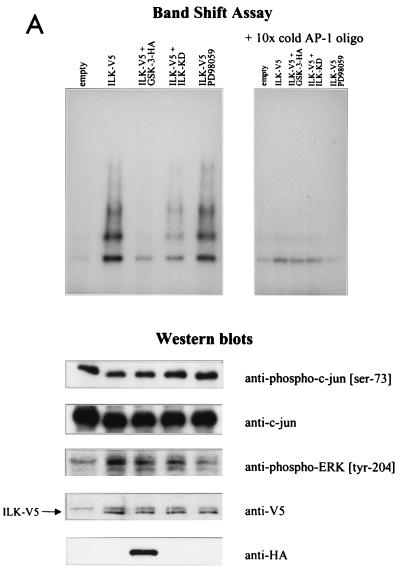
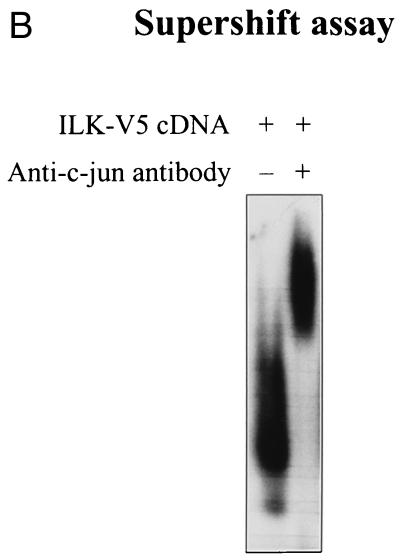
ILK-induced AP-1 activity is mediated by c-jun. (A) In this band shift assay, serum-exposed HEK-293 cells were transfected by Lipofectin with 0.5 μg of each of the indicated cDNAs. PD98059 (25 μM) was added to the medium 12 h prior to harvesting the cells. Nuclear extracts (2 μg) from the transfected cells were incubated with 32P-end-labeled AP-1 consensus oligonucleotide containing the protein binding site. Reaction products were analyzed on a nondenaturing 5% polyacrylamide gel (left gel). The specificity of complex formation was established by a competition experiment using cold AP-1 oligonucleotide as the competitor (right gel). For immunoblot studies, 10 μg of protein was resolved by SDS–10% PAGE. c-jun (Ser-73) phosphorylation, c-jun protein expression level, ERK phosphorylation (Tyr-204), and ERK protein expression level (not shown) were determined by Western blot analysis. ILK and GSK-3 expression levels in the transfected cells were evaluated by Western blot analysis using anti-V5 and anti-HA antibodies, respectively. (B) Anti-c-jun antibody shifts ILK-induced AP-1 complex. Nuclear extracts (2 μg) from HEK-293 cells transfected with ILK-V5 cDNA were incubated with 32P-AP-1 oligonucleotide in the absence or presence of anti-c-jun antibody or nonspecific IgG (10 μg). The latter did not induce a mobility shift of the complex (not shown).