Figure 1.
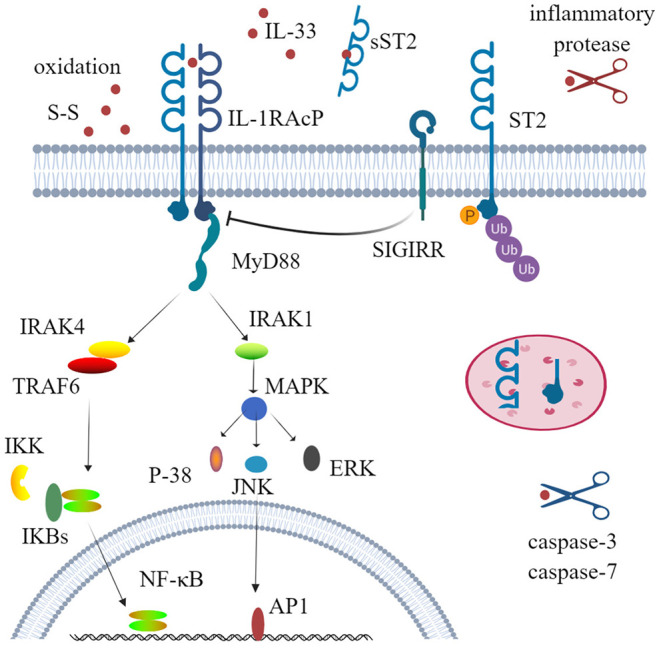
Physiological function and activity regulation of interleukin-33 (IL-33). IL-33 firstly binds to its transmembrane receptor serum stimulation-2 (ST2) to induce conformational changes; then ST2 interacts with IL-1RAcP and recruits the downstream adaptor MyD88, IRAK1, IRAK4, and TRAF6 via Toll/IL-1 receptor domains; and ultimately activates nuclear factor-kappa B (NF-κB) and mitogen-activated protein kinases (ERK, p38, and JNK). This signal pathway can also be inhibited by phosphorylation and ubiquitylation of ST2 and SIGIRR, which disrupt ST2 and IL-1RAcP dimerization. Several mechanisms are involved to regulate the activity of IL-33. Processing by inflammatory proteases can greatly increase (up to 30-fold) cytokine activity, while caspase-3 and caspase-7 lead to cytokine inactivity. Decoy receptor soluble ST2 (sST2) and rapid oxidation of IL-33 are also crucial mechanisms to limit the activity of IL-33. SIGIRR, single immunoglobulin domain IL-1 receptor-related molecule; IRAK, IL-1R-associated kinase; TRAF6, TNF receptor-associated factor 6; ERK, extracellular signal-regulated kinase.
