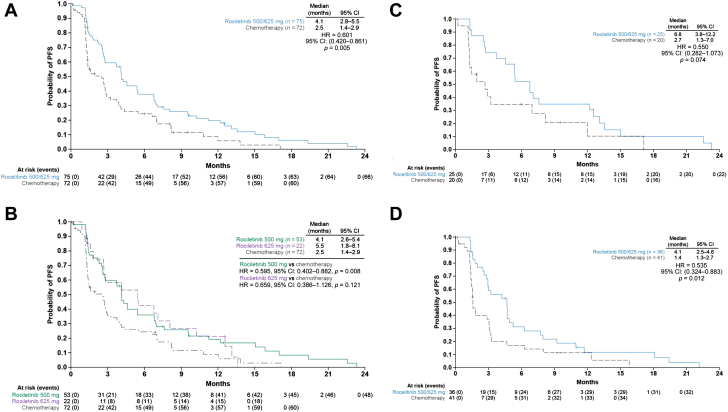
Figure 1.
Investigator-assessed PFS. (A) Rociletinib (500 mg and 625 mg twice daily doses) versus chemotherapy (efficacy population, n = 148).a(B) Rociletinib 625 mg twice daily or 500 mg twice daily versus chemotherapy (efficacy population). (C) Rociletinib (500-mg and 625-mg twice-daily doses) versus chemotherapy in T790M-positive patients; and (D) Rociletinib (500-mg and 625-mg twice-daily doses) versus chemotherapy in T790M-negative patients. aOne patient discontinued because of progressive disease before receiving a single dose of chemotherapy. One patient in the chemotherapy group was not followed up for PFS as the date of their death was not recorded. CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.