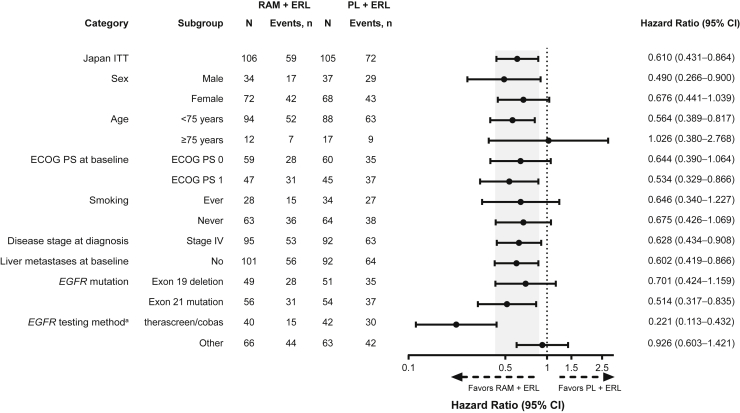
Figure 2.
Investigator-assessed PFS subgroup analysis (Japanese ITT population). The following categories with events less than 10 in either treatment arm are not revealed: unknown smoking history, a disease stage of “other” at diagnosis, and liver metastases at baseline. Shaded area represents the 95% CI for the Japan ITT population. a96% agreement between preplanned confirmatory central EGFR testing (therascreen assay) and local laboratory results (therascreen, cobas, or other PCR and sequencing-based methods) was observed. CI, confidence interval; ECOG PS, Eastern Cooperative Oncology Group performance status; ERL, erlotinib; ITT, intent-to-treat; PCR, polymerase chain reaction; PFS, progression-free survival; PL, placebo; RAM, ramucirumab.