Abstract
Objective
Immune responses are an integral part of the complex reactions to acute cerebral ischemia and contribute to infarct expansion and tissue remodeling. Among damage-associated molecular patterns (DAMPs) the high-mobility group box 1 protein (HMGB1) and calprotectin (S100A8/A9) are released from dying cells and activate the innate immune system.
Methods
To assess DAMPs concentrations and related leukocytic infiltration directly and locally in human stroke patients we performed microcatheter sampling from within the core of the occluded vascular compartment before recanalization by mechanical thrombectomy. These samples from the core of a sealed cerebral-ischemic arterial compartment were compared with systemic control samples from the internal carotid artery obtained after recanalization.
Results
We found increased plasma levels of total free HMGB1 (+33%) and increased S100A8/A9 (+8%) locally within the ischemic cerebral compartment vs. systemic levels. Local concentrations of HMGB1 were associated with more extensive structural brain infarction on admission. In addition, local ischemic HMGB1 and S100A8/A9 concentrations were associated with the numbers of leukocytes that infiltrate the occluded compartment by collateral pathways.
Conclusion
This is the first direct human observation of a local increase in DAMPs concentrations in a uniquely sealed vascular compartment of the ischemic cerebral circulation. These data provide an important pathophysiological link between ischemia-induced cell death and stroke-related inflammation.
Keywords: Danger-associated molecular patterns, High-mobility group box 1 protein, Calprotectin, Hyper-acute stroke
Highlights
-
-
HMGB1 is locally released during occlusion in human hyper-acute stroke
-
-
S100A8/A9 is locally released during occlusion in human hyper-acute stroke
-
-
HMGB1 concentrations are associated with structural brain infarction on admission
-
-
DAMPs concentrations are related to inflammatory responses
1. Introduction
Increasing experimental evidence implies a significant contribution of inflammation to cerebral injury during early ischemia (Stoll and Nieswandt, 2019). However, the molecular triggers that initiate stroke-evoked inflammatory responses are largely unknown, thus hampering the translation of pathophysiological understanding and intervention from experimental models to the human system. High-mobility group box 1 protein (HMGB1) and calprotectin (S100A8/A9) are damage-associated molecular patterns (DAMPs) which are passively released from dying cells or actively secreted by macrophages and many other cell types during injury or severe stress and can profoundly activate the innate immune system (Gong et al., 2020). In the experimental setting, DAMPs are increasingly recognized to modulate cerebral inflammation and brain injury (Ye et al., 2019). Accordingly, increased systemic levels of DAMPs are related to worse functional outcome in human ischemic stroke (IS) (Ye et al., 2019; Guo et al., 2020; Wang et al., 2020). However, the local cerebral concentrations of DAMPs directly at the site of ischemic injury have never been assessed in human IS patients. This was attempted in the current study within the brain vascular territory that during hyperacute stroke is under the occlusion condition. We and others established the technique of microcatheter aspiration of local cerebral-ischemic blood samples from an arterial compartment entirely deprived of antegrade blood flow and immediately before it is re-opened by mechanical thrombectomy (MT) (Kollikowski et al., 2020; Fraser et al., 2019). This method was used to quantify the local infiltration of immune cells into the deep center of the ischemic field by collateral vascular pathways. At the same location, we measured local DAMPs concentration levels of total free HMGB1 and S100A8/A9.
As main finding, we here report with strong statistical confidence the local increase of HMGB1 and S100A8/A9 plasma levels. DAMPs concentrations as represented by these two molecular markers were related to the extent of local immune cell recruitment.
2. Patients and methods
From August 2018 to Mai 2020, we attempted local microcatheter sampling of cerebral-ischemic arterial blood in 258 consecutive patients undergoing emergency MT due to acute large-vessel-occlusion stroke of the anterior circulation. Citrate–phosphate–dextrose–adenine-1 (CPDA) anticoagulated blood from within the cerebral ischemic arterial compartment and systemic control samples from the cervical internal carotid artery (controlled for uncompromised flow conditions) were drawn as described previously (Kollikowski et al., 2020). Briefly, MT by stent-embolus retrieval of angiographically fully occlusive lesions (distal internal carotid artery, middle cerebral artery M1 and proximal M2 segment) was preceded by microcatheter navigation into the midinsular middle cerebral artery M2 segment. After microcatheter positioning and discarding the microcatheter-specific dead space volume, a sample of 1 ml of cerebral-ischemic blood was drawn if possible. Systemic control samples were obtained analogously after embolus removal and/or termination of the thrombectomy procedure. A flow-chart of patient inclusion without missing cases is provided in the appendix (supplemental material, Fig. S1). The cohort of consecutive patients meeting all pre-specified criteria comprised 71 patients. The samples were first analyzed for differential leukocyte counts. After centrifugation to obtain cell free samples, plasma was immediately stored at −20°C. Plasma samples were available in n = 61 unselected patients. Plasmatic HMGB1 (limit of detection: 0.2 ng/ml) was quantified by ELISA (IBL-International, Männedorf, Switzerland) according to the manufacturer´s instructions. The concentration of S100A8/A9 (limit of detection: 0.8 ng/ml) was determined by a LEGENDplex® fluorescent bead immunoassay (Biolegend, San Diego, USA).
2.1. Study approval
This prospective observational study was approved by the local ethics committee of the Medical Faculty of the University of Würzburg, Germany (approval # 135/17). Written informed consent was provided by all participants or their legal representatives.
2.2. Statistics
Statistical analyses were performed using Graph Pad Prism 9 (Graph Pad Software, San Diego, USA). Data are given as mean with 95% confidence interval (CI), as median with interquartile range (IQR), or absolute and relative frequency distribution. Univariate regression analysis was performed to identify associations between ischemic target variables. Normal distribution of datasets was tested using the D´Agostino-Pearsons Test. Wilcoxon Rank-Sum-Test was performed to test for significance. P-values <0.05 (two-sided) were considered statistically significant.
2.3. Data sharing statement
For original data requests contact stoll_g@ukw.de.
3. Results and discussion
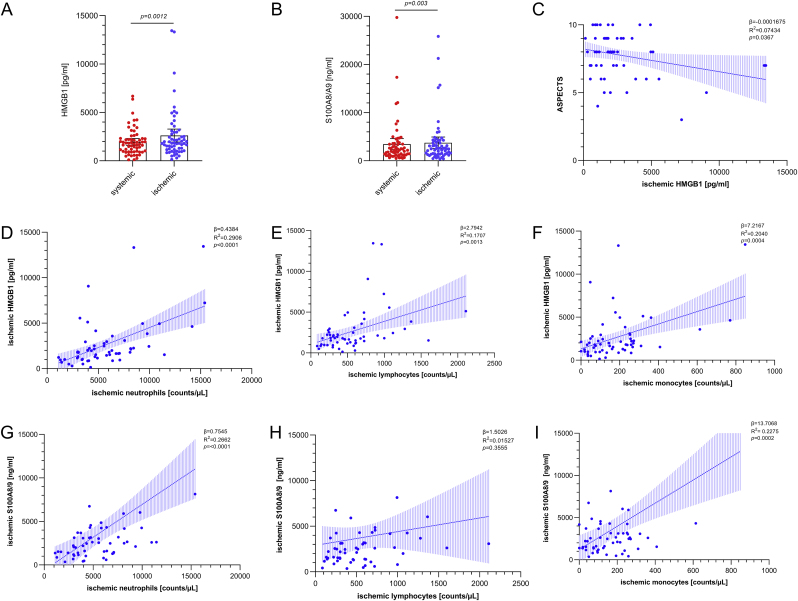
Quantification of HMGB1, S100A8/A9 concentrations, and differential local immune cell counting was performed in a total of 122 samples of 61 unselected patients that met all pre-specified inclusion criteria (Kollikowski et al., 2020). Patient characteristics are shown in Table 1. The median age of patients was 77 years (IQR 68–83), and 38% were of male gender. The median pre-interventional clinical stroke severity as assessed by the National Institutes of Health Stroke Scale (NIHSS) was 15 (IQR 10–17). The local ischemic plasma levels of HMGB1 (+33%) and S100A8/A9 (+8%) were significantly increased when compared to systemic blood which was sampled after recanalization of the occluding thrombus in the cervical ipsilateral internal carotid artery (Fig. 1 A,B; HMGB1(mean): systemic 1959 pg/ml 95% CI: 1610 to 2307 vs. ischemic 2613 pg/ml 95% CI: 1947 to 3278, p = 0.0012 (A); S100A8/A9 (mean): systemic 3450 ng/ml 95% CI: 2281 to 4620 vs. ischemic 3734 ng/ml 95% CI: 2543 to 4925, p = 0.003 (B)). Correlation analysis (Fig. 1 C–I; supplemental material, Table S1 and Table S2) revealed a positive association of structural infarct extent as determined by the Alberta Stroke Program Early CT Score (ASPECTS) imaging prior to MT and local ischemic HMGB1 concentrations (β = -0.0001675, 95% CI = -0.0003242 to −0.00001073, R2 = 0.07434, p = 0.0367). In addition, cerebral-ischemic infiltration of leukocytes was associated with the local concentrations of HMGB1 and S100A8/A9 (HMGB1: neutrophils: p<0.0001; lymphocytes: p = 0.0013; monocytes: p = 0.0004. S100A8/A9: neutrophils: p<0.0001; lymphocytes: p = 0.3555; monocytes: p = 0.0002).
Table 1.
Demographic, clinical, imaging, interventional, and sampling related characteristics (n = 61).
| Demographics | n = 61 |
|---|---|
| Age [years], median (IQR) | 77 (68–83) |
| Male sex, n (%) | 23 (38%) |
| Medical history | |
| Hypertension, n (%) | 54 (89%) |
| Diabetes mellitus, n (%) | 12 (20%) |
| Hyperlipidemia, n (%) | 19 (31%) |
| Atrial fibrillation, n (%) | 35 (57%) |
| Current smoker, n (%) | 5 (8%) |
| Baseline medication | |
| Antithrombotic medication, n (%) | 30 (49%) |
| Antihypertensive drugs, n (%) | 50 (82%) |
| Clinical characteristics | |
| Systolic blood pressure [mmHg], median (IQR) | 160 (150–180) |
| Diastolic blood pressure [mmHg], median (IQR) | 85 (71–96) |
| Heart rate [min-1], median (IQR) | 80 (69–90) |
| NIHSS at presentation, median (IQR) | 15 (10–17) |
| Unknown time of symptom onset, n (%) | 14 (23%) |
| Qualifying ASPECTS, median (IQR) | 8 (7–9) |
| Treatment | |
| Thrombolysis | |
| IV rt-PA, n (%) | 25 (41%) |
| Intervention | |
| Onset-to-puncture [min], median (IQR) | 255 (170–343) |
| Angiographic occlusion location∗ | |
| M1, n (%) | 44 (72%) |
| M2, n (%) | 14 (23%) |
| ICA, n (%) | 10 (16%) |
| Stent-retrieval maneuvers, median (IQR) | 2 (1–3) |
| Successful recanalization, n (%)† | 54 (89%) |
| Onset-to-recanalization time [min], median (IQR) | 322 (245–389) |
| Sampling | |
| Onset-to-ischemic sampling time [min], median (IQR) | 282 (200–367) |
| Onset-to-systemic sampling time [min], median (IQR) |
325 (261–401) |
| Ischemic-to-systemic sampling time [min], median (IQR) | 33 (18–69) |
ASPECTS, Alberta Stroke Program Early CT score; IV rt-PA, intravenous recombinant tissue plasminogen activator; NIHSS, National Institutes of Health Stroke Scale; ∗Including multiple sites per patient; M1/M2, middle cerebral artery section; ICA, internal carotid artery; †definied as mTICI (modified treatment in cerebral infarction) scale 2b or 3.
Fig. 1.
Local DAMPs concentrations distal to middle cerebral artery occlusion in hyper-acute human stroke are increased, correlate with the extent of tissue injury, and DAMPs release is paralleled by immune cell recruitment. (A, B) Local systemic (red points) and ischemic (blue points) plasma levels (n = 61) of HMGB1 (A) and S100A8/A9 (B). Data are shown as scatter dot plot with mean 95% confidence interval (CI). Wilcoxon Rank-Sum-Test was performed to test for significance. p-values reported are 2-sided with p<0.05 being considered statistically significant. (C) Correlation analysis of ischemic HMGB1 with ASPECTS on admission (n = 61). (D–I) Increased ischemic concentrations of HMGB1 (n = 61) and S100A8/A9 (n = 61) are associated with ischemic neutrophil (D, G), lymphocyte (E, H), and monocyte (F, I) counts. Dashed lines depict the 95% confidence interval of the regression lines. ASPECTS = Alberta Stroke Program Early CT Score; R2 = coefficient of determination; β = regression coefficient. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
We here provide the first human data on an early local DAMPs increase in the ischemic cerebral circulation of IS patients under occlusion condition. These observations are based on a novel pial blood sampling approach by which cellular and molecular responses can be assessed directly within the deep center of the ischemic field before it is reopened by MT (pathological condition of slow/low collateral flow) and by which these data can be compared to systemic arterial control samples obtained under physiological flow conditions (Kollikowski et al., 2020).
DAMPs, involving the cytokine-like nuclear protein HMGB1 and S100A8/A9, are alarming signals set free by dying cells under various conditions including ischemia and hypoxia (Kono and Rock, 2008). In our study, the extent of ischemic brain damage was related to the concentration of local total free HMGB1 (+33%; p = 0.0012). This corresponds to seminal experimental data in the middle-cerebral-artery-occlusion-model (MCAO) in mice, describing a rapid redistribution of HMGB1 from the nucleus of neurons to their cytoplasm from where it is released into the brain parenchyma within 3 h after stroke onset (Qiu et al., 2008; Liesz et al., 2015).
In our study, the accumulation of leukocytes within the ischemic vasculature was correlated with the amount of HMGB1 and S100A8/A9, supporting the notion that inflammation, at least partly, is triggered by local increase of DAMPs concentrations. The functional relevance of HMGB1 in cerebral ischemia is supported by data in mice: Application of HMGB1-neutralizing antibodies ameliorated stroke outcome during MCAO (Liesz et al., 2015). As a note of caution, HMGB1 exists in different redox forms as shown by mass spectrometry and, thereby, can interact with different receptors (Antoine et al., 2014). Further studies, both in experimental animals and human stroke patients, are needed to define specific effects of HMGB1 subforms.
Although a functional contribution of S100 protein-driven neuroinflammation has been reported in the ischemic mouse brain, the cellular source of S100A8/A9 is less clear than the widely established neuronal HMGB1 expression. S100A8 and S100A9 are present in leukocytes, and S100A8/A9 has been reported to induce neutrophil as well as monocyte migration (Ryckman et al., 2003). This is in-line with the local human observation in this study that S100A8/A9 concentrations correlated with local neutrophil (p<0.0001) and monocyte counts (p = 0.0002). Here, we bridge the gap between experimental and human stroke research by providing direct evidence of increased local DAMPs amounts in the human brain. Other studies provided compelling, yet indirect, evidence for a potentially detrimental role of HMGB1 and S100A8/A9 in human stroke: Analysis of venous blood samples revealed that systemic HMGB1 levels rapidly increased within 24 h (Goldstein et al., 2006), were related to infarct size (Liesz et al., 2015), and independently predicted 1-year clinical outcome (Huang et al., 2013). Similarly, Guo et al. (2020) demonstrated that high plasma concentrations of S100A8/A9 were independently associated with an increased risk of adverse clinical stroke outcome at 3 months.
Although the precise signaling pathways from increased DAMPs concentrations to stroke-related neuro-inflammation await further elucidation, our data provide a first step in integrating experimental and human data on ultra-early molecular events in the pathophysiology of stroke. This may pave the way for novel anti-inflammatory treatment strategies to augment efficacy of current treatment strategies that so far remain focused on clot recanalization only.
Funding
Deutsche Forschungsgemeinschaft (project number 374031971 – CRC/TR 240, project number 413657723 – UNION CVD).
Author contributions
MKS, AMK, MP and GS designed the study. MKS, AMK and MB performed experiments. MKS, AMK, AGM, MB, MP, and GS analyzed data, discussed results, and provided scientific input throughout the study. MKS, AMK and GS wrote the paper with input and approval from all authors.
Declaration of competing interest
The authors declare that no conflicts of interest exist.
Acknowledgements
We thank Jörn Feick, Marc Strinitz, Thomas Günthner-Lengsfeld, Yanyan Xiong, and Julian Kunz for their support in data acquisition and patient handling. The authors thank Saskia Moritz, Gabriele Köllner and Susi Hellmig for excellent technical support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100270.
Contributor Information
Michael K. Schuhmann, Email: Schuhmann_M@ukw.de.
Alexander M. Kollikowski, Email: Kollikowsk_A@ukw.de.
Alexander G. März, Email: Maerz_A@ukw.de.
Michael Bieber, Email: Bieber_M@ukw.de.
Mirko Pham, Email: Pham_M@ukw.de.
Guido Stoll, Email: Stoll_G@ukw.de.
Appendix A. Supplementary data
The following is/are the supplementary data to this article:
References
- Antoine D.J., Harris H.E., Andersson U., Tracey K.J., Bianchi M.E. A systematic nomenclature for the redox states of high mobility group box (HMGB) proteins. Mol Med. 2014;20:135–137. doi: 10.2119/molmed.2014.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser J.F., Collier L.A., Gorman A.A. The Blood and Clot Thrombectomy Registry and Collaboration (BACTRAC) protocol: novel method for evaluating human stroke. J. Neurointerventional Surg. 2019;11:265–270. doi: 10.1136/neurintsurg-2018-014118. [DOI] [PubMed] [Google Scholar]
- Goldstein R.S., Gallowitsch-Puerta M., Yang L. Elevated high-mobility group box 1 levels in patients with cerebral and myocardial ischemia. Shock. 2006;25:571–574. doi: 10.1097/01.shk.0000209540.99176.72. [DOI] [PubMed] [Google Scholar]
- Gong T., Liu L., Jiang W., Zhou R. DAMP-sensing receptors in sterile inflammation and inflammatory diseases. Nat. Rev. Immunol. 2020;20:95–112. doi: 10.1038/s41577-019-0215-7. [DOI] [PubMed] [Google Scholar]
- Guo D., Zhu Z., Xu T. Plasma S100a8/A9 concentrations and clinical outcomes of ischemic stroke in 2 independent multicenter cohorts. Clin. Chem. 2020;66:706–717. doi: 10.1093/clinchem/hvaa069. [DOI] [PubMed] [Google Scholar]
- Huang J.M., Hu J., Chen N., Hu M.L. Relationship between plasma high-mobility group box-1 levels and clinical outcomes of ischemic stroke. J. Crit. Care. 2013;28:792–797. doi: 10.1016/j.jcrc.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Kollikowski A.M., Schuhmann M.K., Nieswandt B., Mullges W., Stoll G., Pham M. Local leukocyte invasion during hyperacute human ischemic stroke. Ann. Neurol. 2020;87:466–479. doi: 10.1002/ana.25665. [DOI] [PubMed] [Google Scholar]
- Kono H., Rock K.L. How dying cells alert the immune system to danger. Nat. Rev. Immunol. 2008;8:279–289. doi: 10.1038/nri2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesz A., Dalpke A., Mracsko E. DAMP signaling is a key pathway inducing immune modulation after brain injury. J. Neurosci. 2015;35:583–598. doi: 10.1523/JNEUROSCI.2439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J., Nishimura M., Wang Y. Early release of HMGB-1 from neurons after the onset of brain ischemia. J. Cerebr. Blood Flow Metabol. 2008;28:927–938. doi: 10.1038/sj.jcbfm.9600582. [DOI] [PubMed] [Google Scholar]
- Ryckman C., Vandal K., Rouleau P., Talbot M., Tessier P.A. Proinflammatory activities of S100: proteins S100A8, S100A9, and S100A8/A9 induce neutrophil chemotaxis and adhesion. J. Immunol. 2003;170:3233–3242. doi: 10.4049/jimmunol.170.6.3233. [DOI] [PubMed] [Google Scholar]
- Stoll G., Nieswandt B. Thrombo-inflammation in acute ischaemic stroke - implications for treatment. Nat. Rev. Neurol. 2019;15:473–481. doi: 10.1038/s41582-019-0221-1. [DOI] [PubMed] [Google Scholar]
- Wang J., Jiang Y., Zeng D. Prognostic value of plasma HMGB1 in ischemic stroke patients with cerebral ischemia-reperfusion injury after intravenous thrombolysis. J. Stroke Cerebrovasc. Dis. 2020;29:105055. doi: 10.1016/j.jstrokecerebrovasdis.2020.105055. [DOI] [PubMed] [Google Scholar]
- Ye Y., Zeng Z., Jin T., Zhang H., Xiong X., Gu L. The role of high mobility group box 1 in ischemic stroke. Front. Cell. Neurosci. 2019;13:127. doi: 10.3389/fncel.2019.00127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.