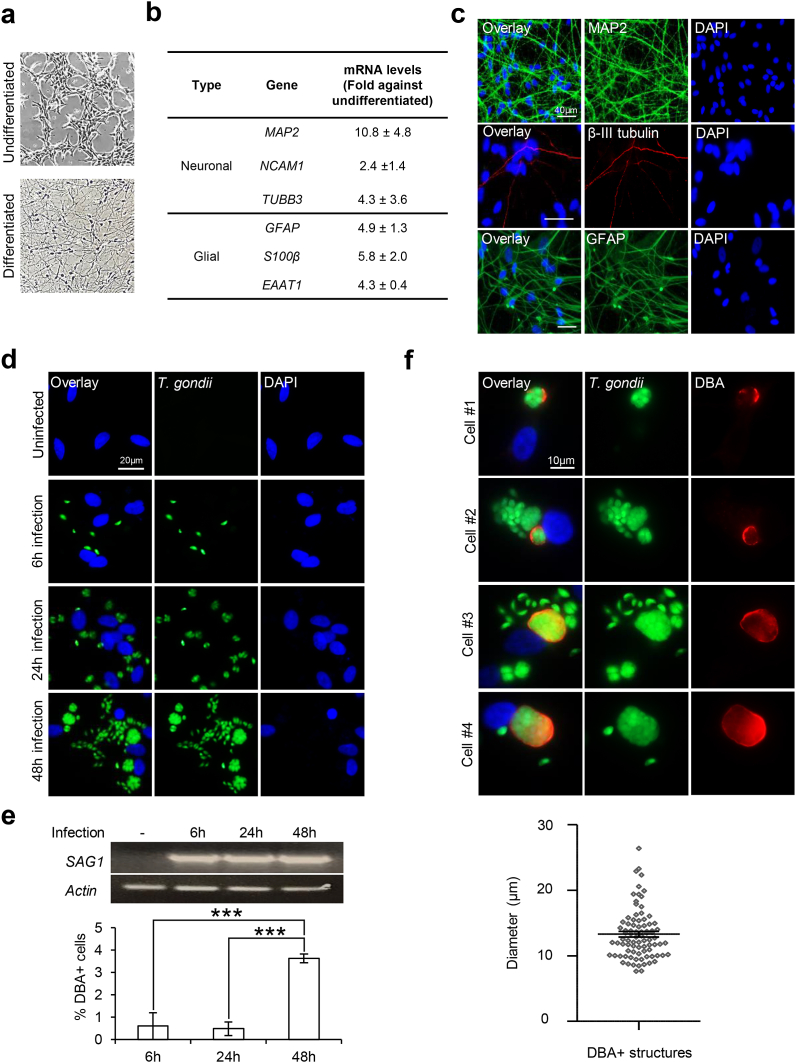
Fig. 1.
T. gondii proliferation and cystdevelopment in differentiated ReNcell neural cells. (a–c) Differentiation of human ReNcell VM neural progenitors upon removal of growth factors for 4 weeks. (a) Bright-field images of undifferentiated and differentiated ReNcell. (b) qPCR analysis of neuronal and glial cell markers in differentiated ReNcell. mRNA levels for each gene were normalized against β-actin and calculated as fold change against the undifferentiated control cells (n = 4–6). (c) Immunofluorescence images of MAP2, β-III tubulin, and GFAP in differentiated ReNcell. (d–f) 3-weeks differentiated ReNcell were infected with GFP-Pru T. gondii at MOI 1 for 6, 24, and 48 h. (d) Fluorescence images of GFP + T. gondii signals. (e) PCR for gene expression of SAG1 (top) and percentage of cells with DBA + structure (bottom). 100–600 cells per experimental set (n = 6) were analyzed for the presence of DBA structure. (f) Representative images of 4 selected cells stained positive for DBA (top) and the diameter of DBA + structures (bottom) at 48 h infection. A total of 84 DBA + structures (one dot represents one structure) identified across 6 experimental sets (n = 6) were analyzed. The horizontal line represents the average diameter. Nuclei were stained with DAPI. All values are mean ± S.E.M. One-way ANOVA with Tukey’s multiple comparison test was performed. Differences against 48 h are significant for ∗∗∗p ≤ 0.0001. Scale bars: 10 μm (Fig. f), 20 μm (Fig. d), or 40 μm (Fig. c).