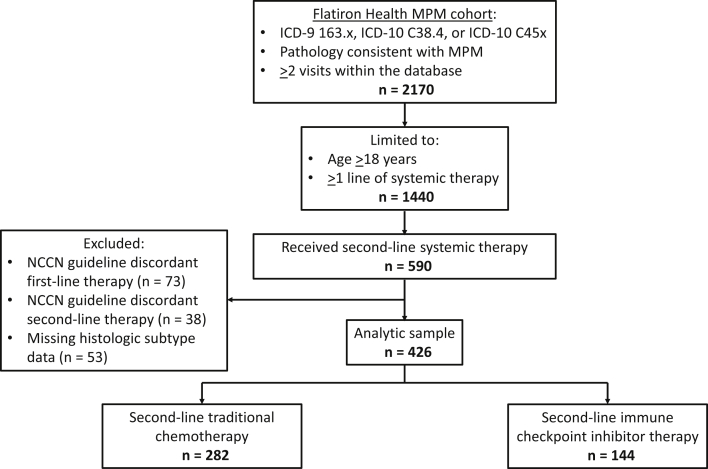
Figure 1.
Assembly of the analytical sample. From the Flatiron Health MPM cohort, the analytical sample was created by selecting adult patients who received at least two lines of systemic therapy and excluding those who received NCCN guideline-discordant first- or second-line therapies and those who had missing MPM histologic subtype data. The analytical sample included 426 patients with MPM who received NCCN guideline-concordant second-line systemic therapy. NCCN guideline-concordant first-line therapies included pemetrexed, carboplatin/cisplatin, bevacizumab, gemcitabine, vinorelbine, and combinations of these medications. NCCN guideline-concordant second-line therapies included traditional chemotherapy (pemetrexed, carboplatin/cisplatin, bevacizumab, gemcitabine, vinorelbine, and combinations of these medications) and immune checkpoint inhibitor therapy (pembrolizumab and nivolumab ± ipilimumab). ICD, International Classification of Diseases; MPM, malignant pleural mesothelioma; NCCN, National Comprehensive Cancer Network.