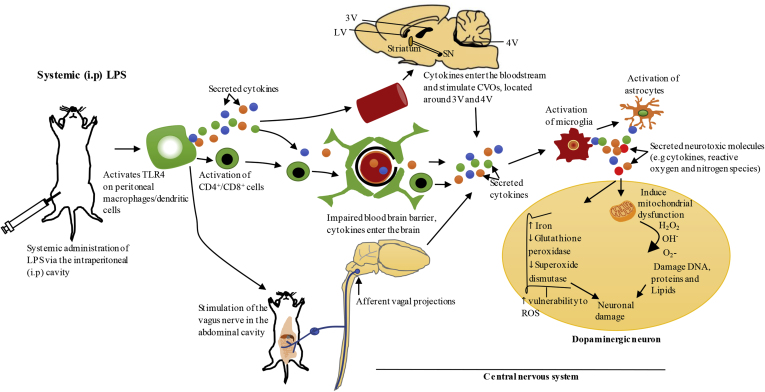
Fig. 2.
Systemic administration of LPS via intraperitoneal cavity-the proposed pathways of propagation of systemic inflammation to the brain.
Systemic administration of LPS via intraperitoneal (i.p) cavity activates Toll-like receptor 4 (TLR4) on peritoneal macrophages/dendritic cells which results in local secretion of inflammatory cytokines such as IL-1, TNF-α and IL-6 (Block et al., 2007; Konsman et al., 1999). Systemic inflammation could be communicated to the brain through several mechanisms. Firstly, IL-1 produced by peritoneal macrophages/dendritic cells at the site of injection in response to systemic LPS, can activate IL-1 receptor on the vagus nerve that innervates the abdominal cavity. This nerve terminates in the nucleus of solitary tract located in the brainstem, which projects to other brain regions that mediate acute sickness response (Konsman et al., 2002; Vitkovic et al., 2000). Secondly, the blood brain barrier (BBB) could lose its integrity under pathological conditions, resulting in the entry of peripheral immune cells such as CD4+/CD8+ T-lymphocytes and inflammatory cytokines into the brain, and these molecules can mediate neuroinflammation through activation of microglia and astrocytes (Hoban et al., 2013). Furthermore, systemic cytokines can communicate with circumventricular organs (CVOs) in the CNS, which are a group of structures located around the third and fourth ventricles that lack BBB, which can also bring about the activation of microglia and astrocytes in these regions. Inflammatory molecules produced in the CVOs could access ventricular cerebrospinal fluid and propagate to other regions of the brain via volume transmission (Perry, 2010; Vitkovic et al., 2000). Exacerbated neuroinflammation in the substantia nigra (SN) could cause degeneration of dopaminergic neurons as outlined in Fig. 1. LV, lateral ventricle; 3V, third ventricle; 4V, fourth ventricle; ROS, reactive oxygen species.