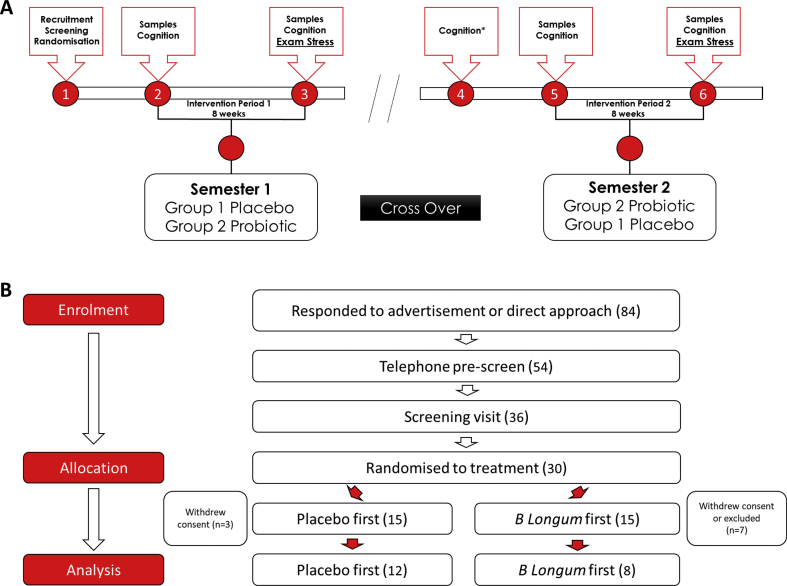
Fig. 1.
Overview of Probiotic Intervention in Chronic Stress.
(A) Visit number is denoted by red circles, visit 1, participants gave informed consent and were recruited to the study and randomised to either a placebo or probiotic group. Visit 2, stool, hair, blood and saliva samples were taken before an 8-week intervention period on placebo or probiotic followed by visit 3, the end of semester 1 visit where stool, hair, blood and saliva samples were obtained. N=9 withdrew consent prior to commencing on the intervention product, and n=1 participant withdrew consent during the first intervention phase due to unwillingness to attend further study visits mainly due to scheduling difficulties. All participants switched intervention for semester 2 which commenced with visit 5 where stool, hair, blood and saliva samples were taken before the 2nd 8-week intervention period. Visit 6 took place at the end of the 8-week intervention where once again, stool, hair, blood and saliva samples were taken from each participant. Semester one and two were conducted based on the exam schedule of the volunteers. Full details of each study visit are in Table 2. (B) Study recruitment, 84 volunteers responded to advertisement and direct contact; 54 were pre-screened; 36 were invited to a screening visit; and thirty were enrolled in the study and randomised to treatment. Following treatment assignment, 3 withdrew from the placebo group and 7 withdrew from the probiotic group. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)