Abstract
Patients with chronic wounds often have associated cognitive dysfunction and depression with an as yet unknown mechanism for this association. To address the possible causality of skin wounding inducing these changes, behavior and cognitive functions of female C57BL/6 mice with an excisional skin wound were compared to unwounded animals. At six days post wounding, animals exhibited anxiety-like behaviors, impaired recognition memory, and impaired coping behavior. Wounded animals also had concomitant increased hippocampal expression of Tnfa, the pattern recognition receptor (PRR) Nod2, the glucocorticoid receptors GR/Nr3c1 and Nr3c2. Prefrontal cortex serotonin and dopamine turnover were increased on day six post-wounding. In contrast to the central nervous system (CNS) findings, day six post -wounding serum catecholamines did not differ between wounded and unwounded animals, nor did levels of the stress hormone corticosterone, TNFα, or TGFβ. Serum IL6 levels were, however elevated in the wounded animals. These findings provide evidence of skin-to-brain signaling, mediated either by elevated serum IL6 or a direct neuronal signaling from the periphery to the CNS, independent of systemic mediators. Wounding in the periphery is associated with an altered expression of inflammatory mediators and PRR genes in the hippocampus, which may be responsible for the observed behavioral deficits.
Keywords: Wounds, Skin-to-Brain signaling, Neuroamines
Highlights
-
•
Mice with skin wounds exhibit altered behavior, memory, and impaired coping.
-
•
Hippocampal neuroinflammation accompanies the behavior aberration.
-
•
Prefrontal cortex serotonin and dopamine turnover are also increased.
-
•
These findings provide evidence of skin-brain signaling in response to wounding.
1. Introduction
The ‘skin-brain axis’ communication network has been recognized as a mechanism by which the central nervous system (CNS) response to psychological stress results in an afferent relay and physiological outcome in the skin (Paus et al., 2006; Pavlovic et al., 2008; Martins et al., 2020; Rodriguez-Vallecillo and Woodbury-Farina, 2014; Chapman and Moynihan, 2009). Stress, through a CNS-mediated mechanism, has been shown to exacerbate peripheral cutaneous manifestations of atopic dermatitis, psoriasis, and even hair growth (Martins et al., 2020; Alexopoulos and Chrousos, 2016; Ayasse et al., 2020; Paus, 2016; Pondeljak and Lugović-Mihić, 2020; Theoharides, 2020; Torales et al., 2020; Yang and Zheng, 2020; Zeiser et al., 2021). However, in addition to the skin being a target for stress responses, bi-directional signaling is described wherein the skin itself, through the localized production of neuroimmunomodulators and stress catecholamines, can initiate stress signaling to the brain (Paus et al., 2006; Chen and Lyga, 2014). The complete implication of this skin-to- brain signaling has not been fully explored.
Here we consider the skin-brain axis as a possible novel paradigm that could reveal the mechanism linking chronic skin wounds to the noted associated cognitive dysfunction. Depression, anxiety and cognitive dysfunction have been associated with chronic skin ulcers of venous or diabetic etiologies (Green et al., 2014; Kouris et al., 2016; Maydick and Acee, 2016; Moffatt et al., 2009; Natovich et al., 2016; Platsidaki et al., 2017; Renner and Erfurt-Berge, 2017; Souza Nogueira et al., 2009; Udovichenko et al., 2017; Zhou and Jia, 2016) (Williams et al., 2010). Neither the mechanism for this association, nor the directional pattern of causality is known. The leading neurobiological mechanism to explain primary mood disorders is the monoamine hypothesis which posits that depression and anxiety are associated with alterations in CNS serotonin, epinephrine and dopamine signaling (Jans et al., 2007; Hillhouse and Porter, 2015). Monoamines, especially dopamine, are essential for memory formation (Wise, 2004) and altered CNS dopamine signaling has been associated with cognitive dysfunction in a number of diseases (Calabresi et al., 2006; Braver and Cohen, 1999; Pan et al., 2019). Additionally, systemic levels of inflammatory cytokines have also been associated with cognitive deficits in patients with post-traumatic stress disorder, and major depressive disease (Hadian et al., 2020; Imai et al., 2018; Roohi et al., 2021). We hypothesized that the affective and cognitive symptoms in subjects with chronic skin wounds associate with alterations in CNS monamines, or with systemic elevation of inflammatory cytokines, and sought to determine the signaling pathways between peripheral wounds and the central nervous system. Therefore, we set out to determine the effect of skin wounding on behavior and identify signaling mechanisms to determine potential causality for the associated findings. Since a large percentage (>40%) of patients with chronic wounds exhibit cognitive disorders that adversely affect their clinical outcome (35) (Gonzalez et al., 2008; Lin et al., 2004; Pearson et al., 2014), finding a link between the skin wound and behavioral abnormalities could explain the observed findings and lead to novel approaches for interrupting this signaling axis.
2. Methods
2.1. Animals
Female C57BL/6 (14–15 week old, 20–24 g) mice were obtained from Jackson Laboratory. Animals were allowed to acclimate for 1 week after transfer from the vendor to the UC Davis vivarium. Mice were housed in cages lined with chip bedding on a 12 h light/dark cycle (lights on at 08:00) with free access to food and water. Animals were kept in the containment unit of the animal facility and all experiments were performed in a biosafety cabinet. All procedures and protocols were reviewed and approved by the institution's IACUC.
2.2. Study design
Mice were randomly assigned to wounded or unwounded groups to avoid bias in study design. Sample size calculation was performed using G∗Power (3.1) based on data collected in our previous behavioral experiments showing impairments, where α = 0.05, power [1-β] = 0.8 (Keogh et al., 2021a, 2021b). Two days prior to wounding surgery, all mice were sedated with 3–5% isoflourane and the dorsal surface was shaved, depilated wounded, and bandaged. Behavioral testing was performed 3 and 6 days post wounding. Control animals were treated identically but not wounded. Despite identical bandaging on control and wounded animals, the wounds on the dorsum of the animals were visible preventing blinding of the behavioral experimenter, who were, however, blind to the experimental outcome during analysis. A subset of videos was analyzed by a second experimenter, unfamiliar with the experimental paradigm, to confirm findings. Analysis of serum, hippocampal and cortical analytes were performed by experimenters blinded to the study design.
2.3. Wounding
Two days prior to wounding surgery, all mice were sedated with 3–5% isoflourane and the dorsal surface was shaved. Depilatory cream was applied and removed within 5–10 s of application to remove remaining hair. For surgery, all mice were anesthetized with isoflourane. Buprenorphine (0.05 mg/kg) was administered subcutaneously prior to wounding procedure. Mice were then sterilely prepped with iodine and ethanol wipes. An 8 mm sterile skin biopsy punch instrument was used to create two bilateral full thickness wounds on the dorsal skin. Skin Shield® (Insight Pharmaceuticals Corp) was applied to the outside edges of each wound to ensure adherence of TegaDerm™ (3 M Medical) to the skin. TegaDerm™ wound dressing was then applied to cover both wounds. Unwounded mice were prepped for surgery and given an identical dose of buprenorphine, but only had Skin Shield ® and TegaDerm ™ applied to their backs without receiving any wound. At the end of the experiment, mice were anesthetized with an overdose of isoflourane. Blood was then drawn via retro-orbital bleeding until totally exsanguinated. Death was then confirmed by cervical dislocation.
2.4. Behavioral testing
Each mouse was subjected to all three tests, with a 1-h rest period between tests. After the last test, mice were allowed to rest for ~1.5 h before sacrifice and tissue collection. The order of tests was the same for each animal, and progressed from least stressful to most; Light/Dark (L/D) Box, then Novel Object Recognition (NOR) Task, then Forced Swim Test (FST). All behavior tests were administered between 7:00am and 1:00pm, and tissue collections happened between 2:30 and 5:00pm. Euthanasia and tissue collection were performed on the day of testing to ensure that the behavior data and biological data were temporally correlated.
2.5. Forced swim test (FST)
The FST was used to assess coping behavior following an inescapable stressor. While not a direct surrogate for depressive-like behavior, impaired FST responses are known to be improved following treatment with anti-depressants (de Kloet and Molendijk, 2016). When placed in a container filled with water, a rodent will make efforts to escape, but eventually will give up and become immobile, which is considered to model behavioral despair. (Yankelevitch-Yahav et al., 2015; Gareau et al., 2011; Emge et al., 2016; Smith et al., 2014). Mice were placed into a 1 L glass beaker with room temperature (20–22C) water and allowed to swim for 6 min with video camera recording of the mouse's activity. The first 2 min of activity were not scored, to exclude the initial adaptation period of the test and the last 4 min of the testing period analyzed manually. During the behavioral analysis, mobility time (any movements other than those necessary to balance the body and keep the head above the water) was measured (Pusceddu et al., 2019).
Female C57BL/6 (14–15 week old, 20–24 g) mice were randomly assigned to experimental groups samples/animals were assigned randomly to various experimental groups (and the specific method of randomization); 2) the data collected was processed randomly and appropriately blocked; 3) experimenters were blind to group assignment and outcome assignment; and 4) an appropriate sample size was computed when the study was being designed.
2.6. Novel object recognition (NOR)Task
Recognition memory was assessed using the highly validated NOR task (Ennaceur and Delacour, 1988). This test is based on the tendency of mice to investigate a novel object in the arena, rather than a known one (Gareau et al., 2011; Ahn et al., 2008). Exploratory behavior was video recorded (5 min) and analyzed for frequency of smell for each object. Objects used included: two metal drawer handles indistinguishable from each other (objects #1 and #2), and a different door handle of similar size, but different in shape and color (object #3). To avoid object bias, preliminary studies tested objects for equal preference. Behavioral assessment consisted of distinct training and testing phases.
2.7. Training (familiarization) phase
Mice were placed individually in a new, clean cage for habituation. After 5 min, objects #1 and #2 were placed in opposite corners of the cage. Behavior was video recorded for 5 min, after which the objects were removed. Mice were rested for 45 min before the (next) testing phase.
2.8. Testing phase
After resting, mice were re-exposed to object #2, which was re-introduced into the cage (referred to now as object #2 B) along with a completely new object (object #3). Behavior was video recorded for 5 min. Memory was assessed as the frequency to explore object #2 B during the testing phase, compared with the new object #3. An exploration ratio was calculated (exploration ratio = freq. smell #3/(freq. smell #3 + freq. smell #2 B) × 100). This ratio represents the proportion of smelling bouts associated with the new object versus the old object. A ratio of 50% represents no discrimination between the two objects and impaired preference. Exploration of objects was defined as orientation towards the object with the nose of the mouse pointed towards the object within 2 cm. The training phase showed no preference between either object #1 or #2.
2.9. Light/dark (L/D) box
Anxiety-like behavior was assessed using the L/D box test (Bourin and Hascoet, 2003). Anxiety-like behavior is assessed as an increased tendency of the animal to remain in the dark box, rather than follow its natural tendency to explore a novel area that is brightly lit (Takao and Miyakawa, 2006). Mice were placed in a clean rat cage lined with bedding containing a light (2/3) and a dark (1/3) compartment. The mouse was introduced to the light compartment and the total time spent in each compartment was recorded by video camera for 10 min. This test lasts a total of 10 min/mouse and is performed once per mouse.
2.10. RNA extraction and purification
Immediately following exsanguination via retro orbital bleed, mice were rapidly decapitated and the whole brain removed for dissection. The cerebellum, hippocampus, and cortex were isolated and snap frozen in liquid nitrogen immediately. 10 mm biopsies including the wound were excised from the dorsal wound and snap frozen in liquid nitrogen immediately after being harvested from the animal. Using RNA Mini Kit (Qiagen, Germantown, MD) tissues were then placed in lysis buffer RLT (Qiagen) with lysis beads, then pulverized using the Bullet Blender (NextAdvance, Troy, NY). RNA was then extracted from the lysate according to the manufacturer's protocol. The resulting total RNA concentration and quality was then assessed via NanoDrop (Thermo Fisher).
2.11. cDNA synthesis and qPCR
100 ng of total RNA was reverse transcribed to cDNA using the High Capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). RNA was then diluted to 100ng/ul and 1 μl was used in each qPCR reaction. Additionally, 5 μl of SsoAdvanced Universal SYBR Green Supermix (Bio-Rad, Hercules, CA), 500 nM forward and reverse primers, and nuclease free water was added to 10 μl per reaction. Changes in gene expression were measured by qPCR using the CFX384 Touch Real-Time PCR Detection System (Bio-Rad, Hercules, CA). Samples were heated to 95 °C for 30 s followed by 40 cycles of 95 °C for 15 s, and 60 °C for 30 s. Genetic expression analysis was performed using the ΔΔCt method and all samples were normalized to the 18s, and Gapdh genes. Reference gene stability was determined to be ideal, and all expression analysis was performed using the CFX Maestro analysis software (Bio-Rad, Hercules, CA). Primers are listed in Supplemental Methods.
2.12. Catecholamine determination
Neurochemicals in mouse cortex and serum were measured using ion-pairing UHPLC with amperometric detection. For all sample types the catecholamines and serotonin, precursors, and metabolites were separated on an Acquity UPLC BEH C18 column, and different mobile phases were prepared for each of the three analyses. Briefly, cortex samples were homogenized in aqueous perchloric acid and proteins were removed by ultrafiltration prior to analysis; serum catecholamines were extracted via phenylboronic acid-silica solid-phase extraction (SPE) and eluted using 1% (m/v) acetic acid prior to analysis; serum tryptophan, serotonin, tryptamine, and 5-HIAA were analyzed after protein precipitation using acidified methanol followed by dilution in 0.1 N HClO4 containing 2 mM EDTA. For cortex neurochemicals and serum catecholamines we performed external standardization and 3,4-dihydroxbenzylamine was added to serum samples as a surrogate standard to account for losses during SPE. For serum tryptamines we performed internal standardization using N-methylserotonin to account for volume and transfer errors as well as variable injection volumes when operating in partial-loop mode. Quantitation was performed using peak areas, or peak area ratios in the case of serum tryptamines, and neurochemical amounts were normalized to tissue mass or serum volume and reported as pmol/gram tissue in cortex or nM for serum measurements. More detailed information is provided in the supplement.
2.13. Serum cytokine detection
Mice were anesthetized with 3% isoflurane and whole blood was extracted via retro orbital draw. The blood was then allowed to clot at room temperature for 15–20 min. The blood was then centrifuged at 1500 g for 10 min to separate serum. A cytometric bead assay was used to determine the serum concentration of TNFα (558299), TGFβ (560429), and IL6 (558301) (BD Biosciences). Assays were performed according to the manufacturers protocol without any changes.
2.14. Serum corticosterone analysis
Blood samples were collected as above for cytokine detection, and serum stored at −80 °C until analysis. Corticosterone levels were assayed using a commercially available enzyme immunoassay (EIA) kit (Corticosterone EIA Kit, ADI-900-097; Enzo Life Sciences, Farmingdale, NY, USA) in accordance with the manufacturer's instructions. Absorbance was read with a multimode plate reader (Synergy H1; BioTek Inc., Winooski, VT, USA) at 405 nm. Results are presented as pg/ml of serum.
2.15. Statistical analysis
Behavior testing data was analyzed with GraphPad Prism 9.0.1 software using the unpaired Mann-Whitney U test. qPCR data was analyzed with Bio-Rad CFX Maestro software and significance set at p < 0.05 using the unpaired T test. For statistical analysis of neurochemicals results, we used Student's t-test for heteroscedastic data. Differences were determined to be significant if p-values were less than 0.05. Pearson's correlation coefficient was used to determine significance for linear correlations. Linear correlations were determined to be significant if the test statistic was larger than the critical value corresponding to p = 0.05 taken from a Student T table.
2.16. Data handling
N numbers: N for each group is listed in each figure legend. Outliers: Outliers were identified using the ROUT (Q = 1%) method in Prism and there were 3 outliers removed from the serum CBA.
2.17. Study approval
All procedures and protocols were reviewed and approved by the institution's IACUC.
3. Results
3.1. Wounded animals develop cognitive and behavioral alterations
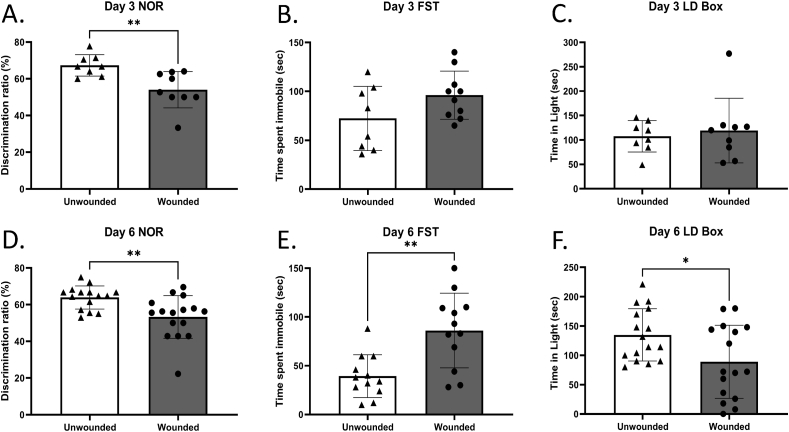
At three days post-wounding the only behavioral impairment identified was impaired recognition memory, identified in the NOR task, as determined by reduced exploration ratio of a novel versus a known object by the wounded animals, compared with sham-treated controls (Fig. 1 A, B, C). By six days post-wounding, however, in addition to decreased recognition memory (Fig. 1D), the wounded animals presented with anxiety-like behavior, as evidenced by decreased total time spent in the light compartment in the L/D box test compared with sham-treated controls (Fig. 1 F). A significant impairment in coping following an inescapable stressor, as evidenced by increased time spent immobile of the wounded animals in the FST was also observed (Fig. 1 E).
Fig. 1.
Wounding results in behavioral deficits.
Animals were either wounded or sham-wounded, and at day 3 (A- C) or 6 (D-F) post-wounding were examined using the novel object recognition (NOR) test (A, D), the forced swim test (FST) (B, E), and the light/dark (L/D) test (C, F). n = 8–10 (Day 3) n = 16 (Day 6). Data are presented as the mean ± SEM, Unpaired, Non Parametric Mann-Whitey test ∗P < 0.05.
3.2. Hippocampal expression of inflammatory mediators is increased in wounded animals
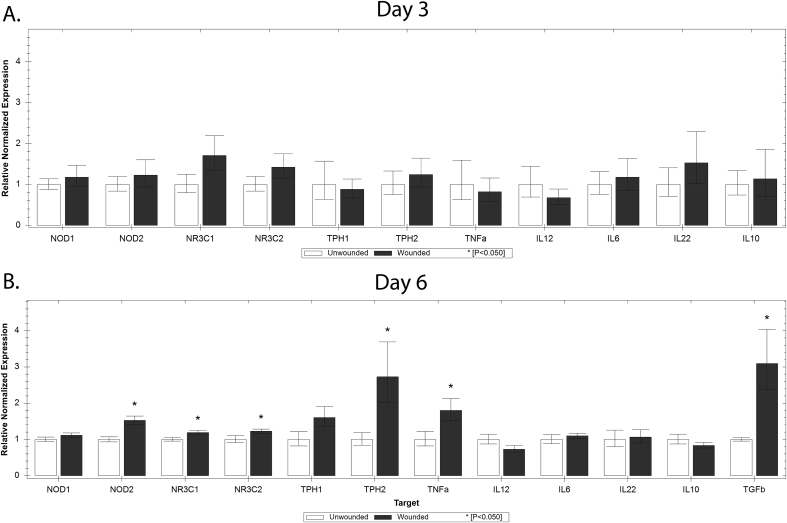
Three days post-wounding there were no significant increases in inflammatory mediators in the hippocampi of wounded animals (Fig. 2 A). However, by day 6 post-wounding, animals demonstrated significant increases in hippocampal expression of the pro-inflammatory cytokine Tnfa the pattern recognition receptor (PRR) Nod2, the glucocorticoid receptors GR/Nr3c1 and Nr3c2. Expression of tryptophan hydroxylase, the rate limiting enzyme for 5HT synthesis, and Tgfb were also increased (Fig. 2 B).
Fig. 2.
Expression of mediators of inflammation in the hippocampus of wounded and unwounded animals.
Gene expression of NOD like receptors 1&2 (Nod1/Nod2), glucocorticoid and mineralcorticoid receptors (Nr3c1/Nr3c2), tryptophan hydroxylase (Tph1/Tph2), tumor necrosis factor alpha (Tnfa), and interleukins 12, 6, 22, and 10 (IL12, IL6, IL22, IL10) 3 days after wounding (Panel A, n = 5 unwounded, 10 wounded), and at day 6 (Panel B, n = 8 unwounded, 16 wounded). Data are presented as relative expression normalized to reference genes ±SEM, ∗P < 0.05.
3.3. IL6 is elevated in serum of wounded animals
To evaluate whether wounding induces an increase in the levels of systemic inflammatory mediators, serum was collected on Days 3 and 6 post-wounding from both wounded and control animals. There were no differences in any of the cytokines measured or corticosterone levels between the wounded and unwounded animals on Day 3 (Supplemental Figure 2). On Day 6, however, IL-6 was elevated in wounded animals relative to the unwounded controls. Interestingly, however, the increase in expression of TGFβ and TNFα in the hippocampus was not mirrored in the serum. The stress hormone, corticosterone was also not elevated in the serum of the wounded animals (Fig. 3).
Fig. 3.
Serum Inflammatory cytokines and corticosterone.
Serum concentrations of TNFα, TGFβ, IL6, and corticosterone 6 days after sham wounding or wounding (n = 15 unwounded, 16 wounded). Data are presented as mean concentration ±SEM, ∗P < 0.05, Unpaired Mann-Whitney test.
3.4. Serotonin and dopamine metabolites are upregulated in cerebral cortex of wounded mice, but not in serum
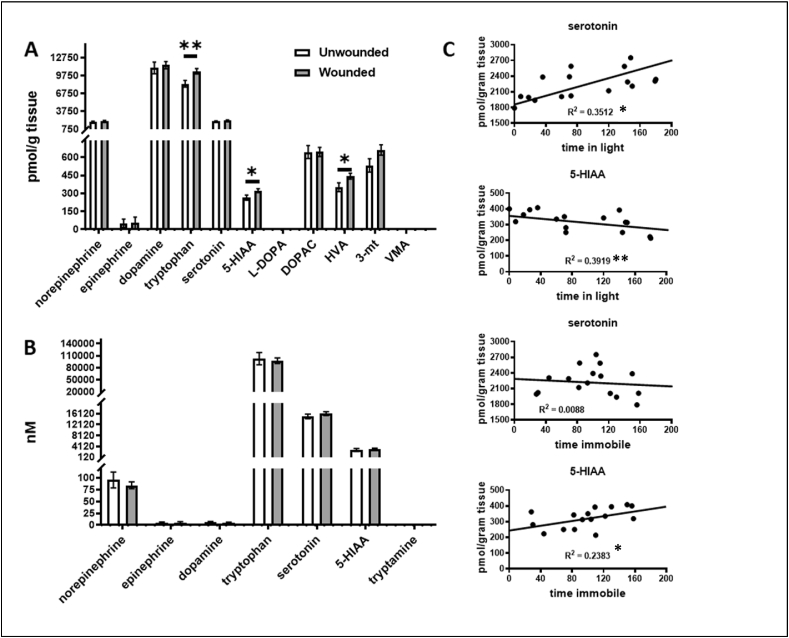
Catecholamines were measured in the cerebral cortices of wounded and sham-wounded animals at days 3 or 6 post-wounding. There was no difference in the levels of neuroamines in either the cortices or serum of wounded vs. unwound animals on day 3 (Supplemental Fig. 1A and B). However, serotonin pathway metabolites were increased in the day 6 wounded animals’ cortices relative to unwounded controls as evidenced by increase in tryptophan precursor and of the serotonin metabolite 5-HIAA (Fig. 4A). The dopamine metabolite, HVA was also elevated in the cortex of wounded animals on day 6 post-wounding (Fig. 4 A). Serum neuroamine metabolites were not significantly different in wounded vs unwounded animals on day 6 post-wounding (Fig. 4B). In wounded animals, the lower levels of 5-HIAA and higher levels of serotonin in the cerebral cortex (Fig. 4C) trended with better performance (more time in the light compartment), while higher levels of 5-HIAA trended with poorer performance on the FST (more time immobile).
Fig. 4.
Cerebral cortex and serum catecholamines in wounded and unwounded animals.
Cerebral cortices of wounded animals on day 6 post wounding demonstrate elevations in dopamine and serotonin pathway members (Panel A). No significant changes in levels of catecholamine neurochemicals noted in sera from wounded vs unwounded animals (Panel B) (n = 15 unwounded, 16 wounded). Data are presented as mean concentration ±SEM, ∗P < 0.05, Student's t-test for heteroscedastic data.
Linear correlations of cerebral cortex serotonin and its 5HIAA metabolite in the wounded animals with behavioral outcomes of the L/D and FST tests (Panel C). R2 is the correlation coefficient. ∗P ≤ 0.050, ∗∗P < 0.01 ≤ 0.01.
4. Discussion
This study demonstrates that skin wounding in mice is associated with the development of memory deficits, anxiety, and coping behavior impairments, indicative of a skin-to-brain signaling axis. While a brain-skin axis has been described decades ago, initial descriptions were unidirectional, with the skin being the target of brain-initiated neuroimmune signaling, contributing to the pathogenesis of allergic and inflammatory skin diseases such as atopic dermatitis or psoriasis (Paus et al., 2006; Buske-Kirschbaum and Hellhammer, 2003; Buske-Kirschbaum et al., 1998). Only more recently has the concept expanded into bi-directional communication along this axis, with demonstration that peripheral, skin-resident nociceptors, when activated by microbial ligands or other noxious stimuli like UV irradiation, can generate signals that are delivered by ascending nerve routes to the brain with resultant neuroimmune responses (Cohen et al., 2019; Slominski et al., 2012; Yang and Chiu, 2017). Here we expand the paradigm of skin-brain bi-directional communication by demonstrating that trauma at the periphery, in the form of excisional wounding of the skin, can induce molecular changes in the brain, and importantly, that these are associated with cognitive and behavioral impairments. Thus, skin wounding is a newly identified noxious stimulus in the periphery that can initiate a skin-brain reflex arc, resulting in behavioral changes.
Behavioral changes and cognitive deficits are common in patients with skin wounds of multiple etiologies, including, venous, arterial, pressure and diabetic foot ulcers, surgical wounds, and other traumatic wounds (Green et al., 2014; Kouris et al., 2016; Moffatt et al., 2009; Natovich et al., 2016; Platsidaki et al., 2017; Renner and Erfurt-Berge, 2017; Souza Nogueira et al., 2009; Udovichenko et al., 2017; Zhou and Jia, 2016; Coelho et al., 2014; Bui et al., 2018; Finlayson et al., 2017; Pedras et al., 2018). Approximately 40% of patients with diabetic skin ulcers exhibit depression (Udovichenko et al., 2017; Pedras et al., 2018), and lower cognitive scores in multiple domains including episodic memory, attention and executive function (Natovich et al., 2016). Likewise there is a 30–40% prevalence of mood disorders in patients with venous ulcers (Souza Nogueira et al., 2009). These associations do not, however, define a causal relationship between the skin ulcer and the observed behavioral change. The current study supports a causal mechanism by demonstrating the association of alterations in brain signaling that could contribute to the observed altered behavior.
The observed associations in expression of hippocampal neuroinflammatory mediators in response to wounding suggest a potential mechanistic link. This is significant because of the implication of hippocampal involvement in neuroplasticity, behavior, and cognition (McKim et al., 2016). The wound-induced increase in hippocampal Tnfa is a hallmark of neuroinflammation, which has been associated with depression, and anxiety-like behavior in rodents (Guo et al., 2020; Wang et al., 2020; Zhang et al., 2020). Similarly, increased Tph2 mRNA (encoding tryptophan hydroxylase 2, the rate-limiting enzyme for brain serotonin, 5-HT biosynthesis) is observed in the dorsal raphe nucleus of rodents exhibiting increased anxiety-like behavior (Donner et al., 2012). We have previously demonstrated an increase in Nod1/2 expression in the hippocampus of adult mice treated with the colitogenic agent dextran sodium sulfate at weaning, which was also associated with cognitive impairments. This hippocampal PRR gene expression was coupled with increased microglia activation and increased cytokine expression suggesting the presence of neuroinflammation (Salvo et al., 2020). Binding of glucocorticoids to the GR is important in sensitizing initial inflammatory responses by the innate immune system, resulting in IL6 production in macrophages in vitro (Busillo et al., 2011). Hippocampal increase in Tgfb expression is reported in association with multiple forms of brain injury (Villapol et al., 2013), and here the novel finding is its increased expression in the brain in response to injury in the periphery, even in the absence of concomitant increase in the serum. Since overexpression of Tgfb in the hippocampus has been shown to result in learning deficits in mice (Martinez-Canabal et al., 2013), it is reasonable to propose that likewise, the wound-induced increase in hippocampal Tgfb potentially contributes to the observed impairment of recognition memory observed in the wounded animals.
Other potential mechanistic links are evident in the observed alteration of the serotonergic and dopaminergic systems in the prefrontal cortex in the wounded animals relative to the controls. In this study we show that wounded mice perform worse on tasks assessing learning (NOR) anxiety-like behaviors (L/D box) and behavioral despair (FST). Wounded mice also had higher levels of metabolites (HVA and 5-HIAA) associated with elevated turnover of the CNS monoamines serotonin and dopamine. CNS dopamine is important for motivation, learning (Cox and Witten, 2019) and memory consolidation (Wise, 2004) which may account for poorer performance in wounded mice on novel learning and behavioral tasks. The L/D box test is an important model for anxiety-like behavior (Bourin and Hascoet, 2003), while antidepressants that modulate serotonin have been shown to improve performance (De Angelis and Furlan, 2000; Hascoet et al., 2000). Among wounded mice, improved performance on L/D box test was associated with higher levels of cerebral serotonin and reduced levels of serotonin breakdown as measured by 5-HIAA. These findings align with monoamine models of mood disorders where serotonin depletion is associated with anxiety (Booij et al., 2003). The FST is a behavioral model to assess coping following an inescapable stress and previously associated with depressive behaviors (Cryan and Holmes, 2005). Performance in the FST is also improved by antidepressants that modulate CNS monoamines (Petit-Demouliere et al., 2005). Among wounded mice, higher levels of 5-HIAA trended towards significance in being associated with worse performance. The monoamine hypothesis of depression continues to inform the most effective treatments for mood disorders that work to modulate and increase CNS serotonin, dopamine and other amine neurotransmitters, transporters and receptors (Jans et al., 2007; Hillhouse and Porter, 2015). Future translational research could consider the utility of common antidepressants that modulate serotonin and dopamine in targeting negative mood symptoms and behaviors among patients with chronic wounds to promote wound healing.
Systemic elevation of IL6 was also observed in day 6 wounded animals. IL6 expression is implicated in pleiotropic responses in cutaneous wounds. In the early phase post-wounding it signals for pro-inflammatory responses to injury, recruiting cytokines and immune cells, and in later phases, IL6 shifts to induce pro-reparative and fibrogenic responses in the anti-inflammatory M2 polarized macrophages, supporting resolution of wound healing. (Johnson et al., 2020), (Bosurgi et al., 2017; Braune et al., 2017), (Wood et al., 2011) Given our findings that serum IL6 was increased at 6 days post-wounding, this would suggest it is involved in regulating wound resolution. There is also extensive documentation of strong correlation of elevated IL6 serum levels in patients with major depressive disorder (Hadian et al., 2020; Roohi et al., 2021). While the exact underpinnings of this association are not completely understood, one documented action of IL6 is the control of the level of the serotonin transporter, and subsequently serotonin uptake (Kong et al., 2015). Thus, the noted increase in systemic levels of IL6 post-wounding could account for the observed increased dopaminergic and serotonergic turnover in the frontal cortex in wounded animals. It is conceivable then, that wound-induced IL6 elevations in the serum influence neurotransmitter metabolism resulting in the observed behavioral changes.
There are some limitations to the current study. Female mice were used to avoid confounding factors that may be attributed sexual dimorphism in wound healing (Gilliver et al., 2008). Thus, future studies should aim to determine if there are sex-based changes in behavior in response to wounding by including male animals. Extended periods of post-wounding evaluation will be needed to determine how long the cognitive alterations persist. The wounds examined in this study do not model human chronic non-healing wounds, since they do heal over time. No one animal model fully reproduces human chronic wounds, to which aging, diabetes, ischemia, immune dysfunction and infection can contribute (Ansell et al., 2012). Future studies will address each of these contributors to wound chronicity and how that may alter cognition. Other factors like bacterial infection in the wound may exacerbate the behavioral changes, much like gut dysbiosis affects cognition and behavior. It is difficult to isolate the possible contribution of pain to the observed behavior results, as pain-mitigating therapeutic approaches induce their own central CNS alterations. Finally, additional studies are needed to fully elucidate the signaling pathway from skin to spinal cord to brain to determine if it is mediated by systemic inflammation and elevation of blood levels of IL6 or a direct signaling pathway from peripheral nociceptors to the CNS.
4.1. Implications for clinical translation
Depression and cognitive impairment are associated with decreased treatment compliance and adherence to self-care tasks (Feil et al., 2012; Gonzalez et al., 2008; Lin et al., 2004; Pearson et al., 2014). Treatment compliance and persistent self-care are critical for optimal wound healing. Patients need to understand how to properly manage their wounds while following recommendations regarding changes in medications and lifestyle habits. When patients are proactively engaged in their treatment, outcomes are improved (Vileikyte et al., 2009). Thus, understanding the pathogenesis of the associated cognitive deficits and depressive behaviors in chronic wound patients can potentially improve their wound outcomes.
5. Conclusion
Skin wounding results in behavioral aberrations, including cognitive impairments, anxiety-like behavior and coping deficits, coupled with associated molecular changes in the brain. These behavioral deficits may indicate a directional skin-to-brain signaling pathway, or an indirect mechanism, stemming from wound-induced elevations of systemic IL6. In either case, these findings expand the current paradigm of bi-directional signaling along this axis. This new understanding of how skin wounds may detrimentally affect the brain and behavior, may provide novel insights on potential targets to disrupt the dysregulation of the skin-brain axis following wounding.
Author contributions
DRF: conducting experiments, acquiring data, analyzing data.
YH: conducting experiments, acquiring data.
ACG: acquiring data, analyzing data.
DD: conducting experiments.
JM: acquiring data.
IK: writing the manuscript.
MGG: conceiving and designing the analysis, writing the manuscript.
RRI: conceiving and designing the analysis, writing the manuscript.
Declaration of competing interest
The Authors declare that there is no conflict of interest.
Acknowledgements
The study was funded in part by NIH grants AR076861 to RRI and 1R01AT009365 to MGG.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbih.2021.100279.
Contributor Information
Melanie G. Gareau, Email: mgareau@ucdavis.edu.
R. Rivkah Isseroff, Email: rrisseroff@ucdavis.edu.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Ahn H.J., Hernandez C.M., Levenson J.M., Lubin F.D., Liou H.C., Sweatt J.D. c-Rel, an NF-kappaB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learn. Mem. 2008;15(7):539–549. doi: 10.1101/lm.866408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexopoulos A., Chrousos G.P. Stress-related skin disorders. Rev. Endocr. Metab. Disord. 2016;17(3):295–304. doi: 10.1007/s11154-016-9367-y. [DOI] [PubMed] [Google Scholar]
- Ansell D.M., Holden K.A., Hardman M.J. Animal models of wound repair: are they cutting it? Exp. Dermatol. 2012;21(8):581–585. doi: 10.1111/j.1600-0625.2012.01540.x. [DOI] [PubMed] [Google Scholar]
- Ayasse M.T., Buddenkotte J., Alam M., Steinhoff M. Role of neuroimmune circuits and pruritus in psoriasis. Exp. Dermatol. 2020;29(4):414–426. doi: 10.1111/exd.14071. [DOI] [PubMed] [Google Scholar]
- Booij L., Van der Does A.J., Riedel W.J. Monoamine depletion in psychiatric and healthy populations: review. Mol. Psychiatr. 2003;8(12):951–973. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- Bosurgi L., Cao Y.G., Cabeza-Cabrerizo M., Tucci A., Hughes L.D., Kong Y. Macrophage function in tissue repair and remodeling requires IL-4 or IL-13 with apoptotic cells. Science. 2017;356(6342):1072–1076. doi: 10.1126/science.aai8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourin M., Hascoet M. The mouse light/dark box test. Eur. J. Pharmacol. 2003;463(1–3):55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- Braune J., Weyer U., Hobusch C., Mauer J., Bruning J.C., Bechmann I. IL-6 regulates M2 polarization and local proliferation of adipose tissue macrophages in obesity. J. Immunol. 2017;198(7):2927–2934. doi: 10.4049/jimmunol.1600476. [DOI] [PubMed] [Google Scholar]
- Braver T.S., Cohen J.D. Dopamine, cognitive control, and schizophrenia: the gating model. Prog. Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Bui U.T., Finlayson K., Edwards H. Risk factors for infection in patients with chronic leg ulcers: a survival analysis. Int. J. Clin. Pract. 2018;72(12) doi: 10.1111/ijcp.13263. [DOI] [PubMed] [Google Scholar]
- Busillo J.M., Azzam K.M., Cidlowski J.A. Glucocorticoids sensitize the innate immune system through regulation of the NLRP3 inflammasome. J. Biol. Chem. 2011;286(44):38703–38713. doi: 10.1074/jbc.M111.275370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buske-Kirschbaum A., Hellhammer D.H. Endocrine and immune responses to stress in chronic inflammatory skin disorders. Ann. N. Y. Acad. Sci. 2003;992:231–240. doi: 10.1111/j.1749-6632.2003.tb03153.x. [DOI] [PubMed] [Google Scholar]
- Buske-Kirschbaum A., Jobst S., Hellhammer D.H. Altered reactivity of the hypothalamus-pituitary-adrenal axis in patients with atopic dermatitis: pathologic factor or symptom? Ann. N. Y. Acad. Sci. 1998;840:747–754. doi: 10.1111/j.1749-6632.1998.tb09613.x. [DOI] [PubMed] [Google Scholar]
- Calabresi P., Picconi B., Parnetti L., Di Filippo M. A convergent model for cognitive dysfunctions in Parkinson's disease: the critical dopamine-acetylcholine synaptic balance. Lancet Neurol. 2006;5(11):974–983. doi: 10.1016/S1474-4422(06)70600-7. [DOI] [PubMed] [Google Scholar]
- Chapman B.P., Moynihan J. The brain-skin connection: role of psychosocial factors and neuropeptides in psoriasis. Expet Rev. Clin. Immunol. 2009;5(6):623–627. doi: 10.1586/eci.09.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Lyga J. Brain-skin connection: stress, inflammation and skin aging. Inflamm. Allergy - Drug Targets. 2014;13(3):177–190. doi: 10.2174/1871528113666140522104422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho C.R., Zantut-Wittmann D.E., Parisi M.C. A cross-sectional study of depression and self-care in patients with type 2 diabetes with and without foot ulcers. Ostomy/Wound Manag. 2014;60(2):46–51. [PubMed] [Google Scholar]
- Cohen J.A., Edwards T.N., Liu A.W., Hirai T., Jones M.R., Wu J. Cutaneous TRPV1(+) neurons trigger protective innate type 17 anticipatory immunity. Cell. 2019;178(4):919–932 e14. doi: 10.1016/j.cell.2019.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Witten I.B. Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci. 2019;20(8):482–494. doi: 10.1038/s41583-019-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan J.F., Holmes A. The ascent of mouse: advances in modelling human depression and anxiety. Nat. Rev. Drug Discov. 2005;4(9):775–790. doi: 10.1038/nrd1825. [DOI] [PubMed] [Google Scholar]
- De Angelis L., Furlan C. The anxiolytic-like properties of two selective MAOIs, moclobemide and selegiline, in a standard and an enhanced light/dark aversion test. Pharmacol. Biochem. Behav. 2000;65(4):649–653. doi: 10.1016/s0091-3057(99)00237-3. [DOI] [PubMed] [Google Scholar]
- de Kloet E.R., Molendijk M.L. Coping with the forced swim stressor: towards understanding an adaptive mechanism. Neural Plast. 2016;2016 doi: 10.1155/2016/6503162. 6503162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner N.C., Johnson P.L., Fitz S.D., Kellen K.E., Shekhar A., Lowry C.A. Elevated tph2 mRNA expression in a rat model of chronic anxiety. Depress. Anxiety. 2012;29(4):307–319. doi: 10.1002/da.21925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emge J.R., Huynh K., Miller E.N., Kaur M., Reardon C., Barrett K.E. Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310(11):G989–G998. doi: 10.1152/ajpgi.00086.2016. [DOI] [PubMed] [Google Scholar]
- Ennaceur A., Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: behavioral data. Behav. Brain Res. 1988;31(1):47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- Feil D.G., Zhu C.W., Sultzer D.L. The relationship between cognitive impairment and diabetes self-management in a population-based community sample of older adults with Type 2 diabetes. J. Behav. Med. 2012;35(2):190–199. doi: 10.1007/s10865-011-9344-6. [DOI] [PubMed] [Google Scholar]
- Finlayson K., Miaskowski C., Alexander K., Liu W.H., Aouizerat B., Parker C. Distinct wound healing and quality-of-life outcomes in subgroups of patients with venous leg ulcers with different symptom cluster experiences. J. Pain Symptom Manag. 2017;53(5):871–879. doi: 10.1016/j.jpainsymman.2016.12.336. [DOI] [PubMed] [Google Scholar]
- Gareau M.G., Wine E., Rodrigues D.M., Cho J.H., Whary M.T., Philpott D.J. Bacterial infection causes stress-induced memory dysfunction in mice. Gut. 2011;60(3):307–317. doi: 10.1136/gut.2009.202515. [DOI] [PubMed] [Google Scholar]
- Gilliver S.C., Ruckshanthi J.P., Hardman M.J., Nakayama T., Ashcroft G.S. Sex dimorphism in wound healing: the roles of sex steroids and macrophage migration inhibitory factor. Endocrinology. 2008;149(11):5747–5757. doi: 10.1210/en.2008-0355. [DOI] [PubMed] [Google Scholar]
- Gonzalez J.S., Peyrot M., McCarl L.A., Collins E.M., Serpa L., Mimiaga M.J. Depression and diabetes treatment nonadherence: a meta-analysis. Diabetes Care. 2008;31(12):2398–2403. doi: 10.2337/dc08-1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green J., Jester R., McKinley R., Pooler A. The impact of chronic venous leg ulcers: a systematic review. J. Wound Care. 2014;23(12):601–612. doi: 10.12968/jowc.2014.23.12.601. [DOI] [PubMed] [Google Scholar]
- Guo X., Zhao Y., Huang F., Li S., Luo M., Wang Y. Effects of transcutaneous auricular vagus nerve stimulation on peripheral and central tumor necrosis factor Alpha in rats with depression-chronic somatic pain comorbidity. Neural Plast. 2020;2020:8885729. doi: 10.1155/2020/8885729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadian Y., Fregoso D., Nguyen C., Bagood M.D., Dahle S.E., Gareau M.G. Microbiome-skin-brain axis: a novel paradigm for cutaneous wounds. Wound Repair Regen. : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2020;28(3):282–292. doi: 10.1111/wrr.12800. [DOI] [PubMed] [Google Scholar]
- Hascoet M., Bourin M., Nic Dhonnchadha B.A. The influence of buspirone, and its metabolite 1-PP, on the activity of paroxetine in the mouse light/dark paradigm and four plates test. Pharmacol. Biochem. Behav. 2000;67(1):45–53. doi: 10.1016/s0091-3057(00)00293-8. [DOI] [PubMed] [Google Scholar]
- Hillhouse T.M., Porter J.H. A brief history of the development of antidepressant drugs: from monoamines to glutamate. Exp. Clin. Psychopharmacol. 2015;23(1):1–21. doi: 10.1037/a0038550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imai R., Hori H., Itoh M., Lin M., Niwa M., Ino K. Inflammatory markers and their possible effects on cognitive function in women with posttraumatic stress disorder. J. Psychiatr. Res. 2018;102:192–200. doi: 10.1016/j.jpsychires.2018.04.009. [DOI] [PubMed] [Google Scholar]
- Jans L.A., Riedel W.J., Markus C.R., Blokland A. Serotonergic vulnerability and depression: assumptions, experimental evidence and implications. Mol. Psychiatr. 2007;12(6):522–543. doi: 10.1038/sj.mp.4001920. [DOI] [PubMed] [Google Scholar]
- Johnson B.Z., Stevenson A.W., Prele C.M., Fear M.W., Wood F.M. The role of IL-6 in skin fibrosis and cutaneous wound healing. Biomedicines. 2020;8(5) doi: 10.3390/biomedicines8050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh C.E., Kim D.H.J., Pusceddu M.M., Knotts T.A., Rabasa G., Sladek J.A. Myelin as a regulator of development of the microbiota-gut-brain axis. Brain Behav. Immun. 2021;91:437–450. doi: 10.1016/j.bbi.2020.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keogh C.E., Rude K.M., Gareau M.G. Role of pattern recognition receptors and the microbiota in neurological disorders. J. Physiol. 2021;599(5):1379–1389. doi: 10.1113/JP279771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong E., Sucic S., Monje F.J., Savalli G., Diao W., Khan D. STAT3 controls IL6-dependent regulation of serotonin transporter function and depression-like behavior. Sci. Rep. 2015;5:9009. doi: 10.1038/srep09009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouris A., Armyra K., Christodoulou C., Sgontzou T., Karypidis D., Kontochristopoulos G. Quality of life psychosocial characteristics in Greek patients with leg ulcers: a case control study. Int. Wound J. 2016;13(5):744–747. doi: 10.1111/iwj.12363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin E.H., Katon W., Von Korff M., Rutter C., Simon G.E., Oliver M. Relationship of depression and diabetes self-care, medication adherence, and preventive care. Diabetes Care. 2004;27(9):2154–2160. doi: 10.2337/diacare.27.9.2154. [DOI] [PubMed] [Google Scholar]
- Martinez-Canabal A., Wheeler A.L., Sarkis D., Lerch J.P., Lu W.Y., Buckwalter M.S. Chronic over-expression of TGFbeta1 alters hippocampal structure and causes learning deficits. Hippocampus. 2013;23(12):1198–1211. doi: 10.1002/hipo.22159. [DOI] [PubMed] [Google Scholar]
- Martins A.M., Ascenso A., Ribeiro H.M., Marto J. The brain-skin connection and the pathogenesis of psoriasis: a review with a focus on the serotonergic system. Cells. 2020;9(4) doi: 10.3390/cells9040796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maydick D.R., Acee A.M. Comorbid depression and diabetic foot ulcers. Home Healthc. Nurse. 2016;34(2):62–67. doi: 10.1097/NHH.0000000000000340. [DOI] [PubMed] [Google Scholar]
- McKim D.B., Niraula A., Tarr A.J., Wohleb E.S., Sheridan J.F., Godbout J.P. Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J. Neurosci. 2016;36(9):2590–2604. doi: 10.1523/JNEUROSCI.2394-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moffatt C.J., Franks P.J., Doherty D.C., Smithdale R., Steptoe A. Psychological factors in leg ulceration: a case-control study. Br. J. Dermatol. 2009;161(4):750–756. doi: 10.1111/j.1365-2133.2009.09211.x. [DOI] [PubMed] [Google Scholar]
- Natovich R., Kushnir T., Harman-Boehm I., Margalit D., Siev-Ner I., Tsalichin D. Cognitive dysfunction: Part and parcel of the diabetic foot. Diabetes Care. 2016;39(7):1202–1207. doi: 10.2337/dc15-2838. [DOI] [PubMed] [Google Scholar]
- Pan X., Kaminga A.C., Wen S.W., Wu X., Acheampong K., Liu A. Dopamine and dopamine receptors in alzheimer's disease: a systematic review and network meta-analysis. Front. Aging Neurosci. 2019;11:175. doi: 10.3389/fnagi.2019.00175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus R. Exploring the "brain-skin connection": leads and lessons from the hair follicle. Curr Res Transl Med. 2016;64(4):207–214. doi: 10.1016/j.retram.2016.10.003. [DOI] [PubMed] [Google Scholar]
- Paus R., Theoharides T.C., Arck P.C. Neuroimmunoendocrine circuitry of the 'brain-skin connection. Trends Immunol. 2006;27(1):32–39. doi: 10.1016/j.it.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Pavlovic S., Daniltchenko M., Tobin D.J., Hagen E., Hunt S.P., Klapp B.F. Further exploring the brain-skin connection: stress worsens dermatitis via substance P-dependent neurogenic inflammation in mice. J. Invest. Dermatol. 2008;128(2):434–446. doi: 10.1038/sj.jid.5701079. [DOI] [PubMed] [Google Scholar]
- Pearson S., Nash T., Ireland V. Depression symptoms in people with diabetes attending outpatient podiatry clinics for the treatment of foot ulcers. J. Foot Ankle Res. 2014;7(1):47. doi: 10.1186/s13047-014-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedras S., Carvalho R., Pereira M.G. Predictors of quality of life in patients with diabetic foot ulcer: the role of anxiety, depression, and functionality. J. Health Psychol. 2018;23(11):1488–1498. doi: 10.1177/1359105316656769. [DOI] [PubMed] [Google Scholar]
- Petit-Demouliere B., Chenu F., Bourin M. Forced swimming test in mice: a review of antidepressant activity. Psychopharmacology. 2005;177(3):245–255. doi: 10.1007/s00213-004-2048-7. [DOI] [PubMed] [Google Scholar]
- Platsidaki E., Kouris A., Christodoulou C. Psychosocial aspects in patients with chronic leg ulcers. Wounds. 2017;29(10):306–310. doi: 10.25270/wnds/2017.10.306310. [DOI] [PubMed] [Google Scholar]
- Pondeljak N., Lugović-Mihić L. Stress-induced interaction of skin immune cells, hormones, and neurotransmitters. Clin. Therapeut. 2020;42(5):757–770. doi: 10.1016/j.clinthera.2020.03.008. [DOI] [PubMed] [Google Scholar]
- Pusceddu M.M., Barboza M., Keogh C.E., Schneider M., Stokes P., Sladek J.A. Nod-like receptors are critical for gut-brain axis signalling in mice. J. Physiol. 2019;597(24):5777–5797. doi: 10.1113/JP278640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner R., Erfurt-Berge C. Depression and quality of life in patients with chronic wounds: ways to measure their influence and effect on daily life. Chron. Wound Care Manag. Res. 2017;(4):143–151. [Google Scholar]
- Rodriguez-Vallecillo E., Woodbury-Farina M.A. Dermatological manifestations of stress in normal and psychiatric populations. Psychiatr. Clin. 2014;37(4):625–651. doi: 10.1016/j.psc.2014.08.009. [DOI] [PubMed] [Google Scholar]
- Roohi E., Jaafari N., Hashemian F. On inflammatory hypothesis of depression: what is the role of IL-6 in the middle of the chaos? J. Neuroinflammation. 2021;18(1):45. doi: 10.1186/s12974-021-02100-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvo E., Stokes P., Keogh C.E., Brust-Mascher I., Hennessey C., Knotts T.A. A murine model of pediatric inflammatory bowel disease causes microbiota-gut-brain axis deficits in adulthood. Am. J. Physiol. Gastrointest. Liver Physiol. 2020;319(3) doi: 10.1152/ajpgi.00177.2020. G361-g74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slominski A.T., Zmijewski M.A., Skobowiat C., Zbytek B., Slominski R.M., Steketee J.D. Sensing the environment: regulation of local and global homeostasis by the skin's neuroendocrine system. Adv. Anat. Embryol. Cell Biol. 2012;212:1–115. doi: 10.1007/978-3-642-19683-6_1. vii. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith C.J., Emge J.R., Berzins K., Lung L., Khamishon R., Shah P. Probiotics normalize the gut-brain-microbiota axis in immunodeficient mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014;307(8):G793–G802. doi: 10.1152/ajpgi.00238.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza Nogueira G., Rodrigues Zanin C., Miyazaki M.C., Pereira de Godoy J.M. Venous leg ulcers and emotional consequences. Int. J. Low. Extrem. Wounds. 2009;8(4):194–196. doi: 10.1177/1534734609350548. [DOI] [PubMed] [Google Scholar]
- Takao K., Miyakawa T. Light/dark transition test for mice. JoVE : JoVE. 2006;(1):104. doi: 10.3791/104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theoharides T.C. Effect of stress on neuroimmune processes. Clin. Therapeut. 2020;42(6):1007–1014. doi: 10.1016/j.clinthera.2020.05.002. [DOI] [PubMed] [Google Scholar]
- Torales J., Echeverría C., Barrios I., García O., O'Higgins M., Castaldelli-Maia J.M. Psychodermatological mechanisms of psoriasis. Dermatol. Ther. 2020 doi: 10.1111/dth.13827. [DOI] [PubMed] [Google Scholar]
- Udovichenko O.V., Maximova N.V., Amosova M.V., Yunilaynen O.A., Berseneva E.A., Starostina E.G. Prevalence and prognostic value of depression and anxiety in patients with diabetic foot ulcers and possibilities of their treatment. Curr. Diabetes Rev. 2017;13(1):97–106. doi: 10.2174/1573399812666160523143354. [DOI] [PubMed] [Google Scholar]
- Vileikyte L., Peyrot M., Gonzalez J.S., Rubin R.R., Garrow A.P., Stickings D. Predictors of depressive symptoms in persons with diabetic peripheral neuropathy: a longitudinal study. Diabetologia. 2009;52(7):1265–1273. doi: 10.1007/s00125-009-1363-2. [DOI] [PubMed] [Google Scholar]
- Villapol S., Logan T.T., Symes A.J. In: Trends in Cell Signaling Pathways in Neuronal Fate Decision. Wislet-Gendebien S., editor. IntechOpen; 2013. [Google Scholar]
- Wang L., Guo T., Guo Y., Xu Y. Asiaticoside produces an antidepressantlike effect in a chronic unpredictable mild stress model of depression in mice, involving reversion of inflammation and the PKA/pCREB/BDNF signaling pathway. Mol. Med. Rep. 2020;22(3):2364–2372. doi: 10.3892/mmr.2020.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L.H., Rutter C.M., Katon W.J., Reiber G.E., Ciechanowski P., Heckbert S.R. Depression and incident diabetic foot ulcers: a prospective cohort study. Am. J. Med. 2010;123(8):748–754 e3. doi: 10.1016/j.amjmed.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A. Dopamine, learning and motivation. Nat. Rev. Neurosci. 2004;5(6):483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wood M.W., Breitschwerdt E.B., Gookin J.L. Autocrine effects of interleukin-6 mediate acute-phase proinflammatory and tissue-reparative transcriptional responses of canine bladder mucosa. Infect. Immun. 2011;79(2):708–715. doi: 10.1128/IAI.01102-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N.J., Chiu I.M. Bacterial signaling to the nervous system through toxins and metabolites. J. Mol. Biol. 2017;429(5):587–605. doi: 10.1016/j.jmb.2016.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H., Zheng J. Influence of stress on the development of psoriasis. Clin. Exp. Dermatol. 2020;45(3):284–288. doi: 10.1111/ced.14105. [DOI] [PubMed] [Google Scholar]
- Yankelevitch-Yahav R., Franko M., Huly A., Doron R. The forced swim test as a model of depressive-like behavior. JoVE : JoVE. 2015;(97) doi: 10.3791/52587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeiser K., Hammel G., Kirchberger I., Traidl-Hoffmann C. Social and psychosocial effects on atopic eczema symptom severity - a scoping review of observational studies published from 1989 to 2019. J. Eur. Acad. Dermatol. Venereol. 2021;35(4):835–843. doi: 10.1111/jdv.16950. Epub 2020 Nov 16. [DOI] [PubMed] [Google Scholar]
- Zhang K., Lin W., Zhang J., Zhao Y., Wang X., Zhao M. Effect of Toll-like receptor 4 on depressive-like behaviors induced by chronic social defeat stress. Brain Behav. 2020;10(3) doi: 10.1002/brb3.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou K., Jia P. Depressive symptoms in patients with wounds: a cross-sectional study. Wound Repair Regen. : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2016;24(6):1059–1065. doi: 10.1111/wrr.12484. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.