Abstract
Oxidative stress and inflammation are closely related to atherosclerotic cardiovascular disease. It is established that hydrogen has significant protective effects on many diseases as a potential antioxidative and anti-inflammatory agent. The purpose of this study is to evaluate the effect of hydrogen on unstable angina in vitro and in vivo. An atherosclerosis model in vitro was constructed by ox-LDL-induced injury of human umbilical vein endothelial cells and in vitro testing indicated hydrogen inhibited ox-LDL-induced oxidative stress and inflammatory response by down-regulating LOX-1/NF-kB signaling pathway. Subsequently, the attenuating effect of hydrogen-rich water intake on unstable angina was further confirmed in clinic. Forty hospitalized subjects with unstable angina were enrolled and consumed either 1000–1200 mL/d hydrogen-rich water or the same amount of placebo pure water in addition to conventional drugs for three months. Clinical analysis showed hydrogen-rich water intake relieved angina symptoms in unstable angina patients. Serum analysis showed that hydrogen-rich water addition resulted in more effective reductions of total-cholesterol, low-density lipoprotein-cholesterol, and apolipoprotein B levels compared with conventional treatment. These results support that hydrogen as adjuvant treatment has a beneficial effect on unstable angina.
Keywords: : Hydrogen, unstable angina, atherosclerosis, oxidative stress, inflammation, lectin-like oxidized low-density lipoprotein receptor-1, NF-κB p65
Impact statement
Hydrogen, a natural and powerful antioxidative and anti-inflammatory agent, has significant protective effect on various diseases. Our study demonstrated that hydrogen inhibited ox-LDL-induced oxidative stress and inflammatory response by down-regulating LOX-1/NF-kB signaling pathway in human umbilical vein endothelial cells. More importantly, our clinical trial further confirmed hydrogen-rich water as adjuvant treatment contributed to the easement of angina symptoms and the regulation of serum cholesterol levels for patients with unstable angina. These results suggest hydrogen can be a potential strategy for the treatment of atherosclerotic cardiovascular disease.
Introduction
Cardiovascular disease (CVD) is the leading factor of death around the world, accounting for 30% of all cases of death, among which 42% are resulted from coronary heart disease (CHD). 1 Acute coronary syndrome (ACS) is the serious stage of CHD and acute myocardial infarction and unstable angina (UA) are involved. Despite of the application of contemporary therapies, patients with ACS still face tough risk of early and recurrent cardiovascular events. 2 The potential role of oxidative stress and inflammatory reaction has been indicated in the progression and clinical instability of ACS by increasing the number of studies. 3 Necropsy studies show there are inflammatory cells in atherosclerotic plaque, 4 and clinical outcomes also display a correlation between systemic inflammatory markers and CVD. 5 Therefore, antioxidant and anti-inflammatory therapy is expected to become an important means to prevent and treat ACS.
Hydrogen, the smallest elemental gas molecule, is capable of penetrating into the cytoplasm, mitochondria, and nucleus of cells by simply diffusing. Both hydroxyl radicals and peroxynitrite anions, which are the most destructive reactive oxygen species (ROS), can be selectively scavenged by hydrogen. Therefore, hydrogen has natural and powerful antioxidative and anti-inflammatory functions. It has been demonstrated that hydrogen has significant protective effects on many pathological processes such as ischemia reperfusion, 6 ionizing radiation, 7 and inflammatory injuries. 8 Our previous research showed that intraperitoneal injection of hydrogen-saturated saline effectively mitigated atherosclerotic lesion of apoE−/− mice fed either chow or high-fat diet. 9 In addition, our former study also showed that long-term drinking of hydrogen-rich water (HRW) could better serum lipid composition and lipoprotein function for patients with potential metabolic syndrome. 10 In current study, an in vitro atherosclerosis (AS) model was firstly built by ox-LDL-induced injury of human umbilical vein endothelial cells (HUVECs) to explore the protective effect of hydrogen. Thereafter, a small clinical trial was carried out to further verify whether hydrogen alleviated UA in clinic or not.
Materials and methods
Cell culture
HUVECs (EA.hy926) were from Shanghai Institute of Biochemistry and grew in RPMI-1640 medium (HyClone, China) supplemented with 10% fetal bovine serum (HyClone, China). Cells grown to subconfluence were divided into four groups, namely control, hydrogen, ox-LDL, and ox-LDL + hydrogen group. Cells were respectively put into normal incubator without hydrogen or hydrogen incubator with 50% H2 (Jinan Haowei Experimental Instrument Co., Ltd, China) during the process of cell experiments. After adhesion, the latter two groups were stimulated with 100 μg/mL ox-LDL for 24 h. Cells and supernatants were harvested and relevant indexes were tested.
Flow cytometric assessment of mitochondrial generation of ROS
Mitochondrial generation of ROS, specifically superoxide anion, was evaluated using mitoSOX red kit (KeyGEN biotech Co., China), in which there was a lipid soluble cation. It selectively targeted to the mitochondrial matrix and emitted red fluorescence when oxidized. After treatment, HUVECs were gently washed twice with warm PBS and then covered with 1 mL of 5 µmol/L mitoSOX reagent working solution. The cells were incubated in darkness for 10 min at 37°C. Following two centrifugations and washes, mitoSOX fluorescence intensity was detected by flow cytometry.
Measurement of MDA and SOD
According to the manufacturer’s instructions of kits (Nanjing Jiancheng Bioengineering Institute, China), the levels of malondialdehyde (MDA) in cultured cells in vitro were evaluated by measuring thiobarbituric acid-reactive substances (TBARS). Superoxide dismutase (SOD) activity was also tested by Fridovich’s method, in which xanthine and xanthine oxidase were applied to produce superoxide radicals to react with p-iodonitrotetrazolium violet to generate a red formazan measured at 505 nm. 11
Immunofluorescence assay
Cells were incubated on coverslips. After the corresponding intervention, cells were tenderly washed with PBS for three times, fixed by 4% paraformaldehyde for 20 min and permeabilized by 0.1% Triton-X 100 for 5 min. The coverslips were blocked with 10% donkey serum and incubated with the primary antibody (Abcam, USA) of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), or nuclear factor kappa B (NF-κB) p65 for whole night at 4°C. Then the coverslips were exposed to Alexa Fluor 488-conjugated secondary antibody (Thermo Fisher Scientific, USA) for 1 h at room temperature. After counterstained with DAPI, the coverslips were photographed under a laser scanning confocal microscope (Bio-Rad Radiance 2100).
Western blot
Total protein and nuclear protein from treated cells were extracted using RIPA lysis buffer (Solarbio, China) and nuclear protein extraction kit (BestBio, China), respectively. The equal amounts of protein were subjected to 12% SDS-PAGE and transferred onto PVDF membranes through electroblotting. Then the membranes were blocked with skimmed milk powder and incubated with primary antibodies against lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) and NF-kB p65 (Abcam, USA) for whole night at 4°C. Following washed with TBS containing 0.1% Tween 20, the membranes were incubated with horseradish peroxidase-conjugated secondary antibodies (Zsbio, China) for 2 h at room temperature. Thereafter, the immunoblots were exposed by ECL reaction and visualized using a high-performance chemiluminescence film. The IOD value of immunoreactive bands was calculated by Image-Pro Plus software and normalized by house-keeping protein β-actin or histone H3 (Abcam, USA).
Study population and design
In this study, a randomized-controlled trial was taken for clinical observation. Forty hospitalized individuals with UA from Cardiovascular Department in The Second Affiliated Hospital of Shandong First Medical University were screened. They must meet one of the following three: (1) Recent onset (≤1 month) angina pectoris with ST segment depression or T wave depression or inversion; (2) Angina pectoris at rest; (3) Deterioration of original exertional angina. 12 There was no limitation to patient’s gender, age, occupation, and race. Patients with other viscera disease, viral myocarditis, AMI, diabetes mellitus, mental illness, and related drug allergy were excluded.
Forty participants were divided into HRW group (n = 20) and control group (n = 20) and received a three-month intervention. Control group were given routine treatment and nursing and required them to drink 1000–1200 mL pure water per day; HRW group drank HRW for three months in addition to conventional drugs and its volume per day was also about 1000–1200 mL. HRW was from hydrogen producing cup (Zhishui miniature hydrogen-rich water cup, HW-G2 model, Shuangdi Electronic Technology Co., Ltd, China). The cup can electrolyze water to produce hydrogen. The cup was given to patients two days before discharge and its correct use-method was informed in detail. Considering that hydrogen density was low and it easily volatilized, patients were advised to drink HRW within 30 min and to cover the cup tightly during drinking. Before the intervention and three months later, the clinical parameters of patients were collected and recorded.
In the intervention period, all participants attended 1-h sessions for health education once a month. The low-fat, low-salt, and low-sugar diet were required. The proper exercise and walk for about half an hour every day were recommended. Overstrain and excitable states were not admitted. Diaries of both diet and exercise were completed by participants and reviewed at each visit. The patients were also phoned once a week to increase their adherence to the study protocol.
Standard for reduction of angina symptoms
The standard for efficacy evaluation of angina pectoris was referred from the literature. 13 Markedly effective: angina attack was not caused by equal level of exertion as before, or angina frequency and dosage of nitroglycerin lowered by more than 80%; Effective: angina frequency and dosage of nitroglycerin lowered by 50%–80%; Ineffective: angina frequency and dosage of nitroglycerin lowered by less than 50% or even increased.
Measurement of serum lipid and lipoprotein parameters
At both the beginning and the end of the study, blood samples were taken after 12 to 14 h of fasting state to obtain serum. TC, TG, LDL-C, HDL-C, apoB, and apoA1 in serum were tested using standard laboratory methods in The Second Affiliated Hospital of Shandong First Medical University. The data of patients whose lipid or lipoprotein level were reduced above 20% after three-month intervention was considered to be effective. The effective rate was calculated by the ratio of effective patients’ number to total patients’ number.
Statistical analysis
Statistical analysis was performed by SPSS 24.0 (IBM, USA). The qualitative data were expressed as frequency and percentage, and Chi-square test was utilized to analyze the statistical difference between groups. Quantitative data were presented as mean ± standard deviation, and the data from in vitro experiments were analyzed by one-way analysis of variance (ANOVA) followed by Student–Newmann–Keuls multiple comparison tests. A P-value <0.05 was considered statistically different.
Results
In vitro tests
Hydrogen inhibited ROS production and alleviated oxidative stress in ox-LDL-induced HUVECs
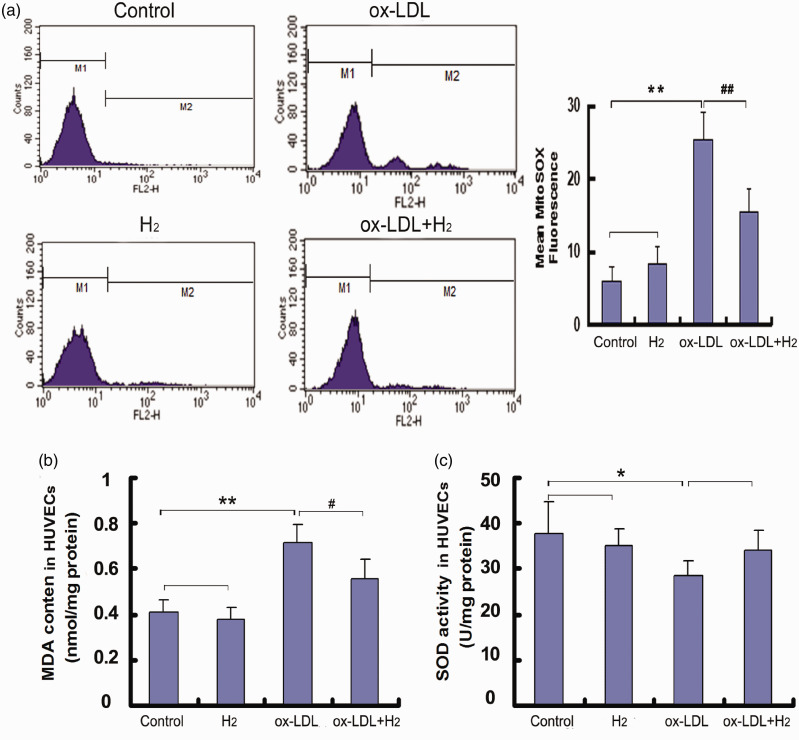
Mitochondria are the important site of ROS production. Therefore, we used a specific mitochondrial superoxide indicator, mitoSOX, to test the effect of hydrogen on mitochondrial ROS generation of live cells. As expected, ox-LDL significantly increased mitoSOX Red fluorescence intensity in HUVECs and hydrogen treatment abolished the increase (Figure 1(a)). In this study, we also determined MDA level and SOD activity in HUVECs. After 24-h exposure of ox-LDL (100 μg/mL), MDA content was enhanced and SOD activity was lowered in HUVECs. However, hydrogen treatment decreased MDA level in ox-LDL-induced HUVEC cells (Figure 1(b)).
Figure 1.
Hydrogen inhibited mitochondrial ROS generation and decreased MDA content in ox-LDL-induced HUVEC cells. The mitochondria-specific ROS level was determined using MitoSOX and quantified by flow cytometry. MDA content and SOD activity in cells were tested using the corresponding kits. (a) Flow cytometry images and quantification of mitoSOX fluorescence; (b) MDA content in HUVECs; (c) SOD activity in HUVECs. Values obtained from three independent experiments were expressed as mean ± SD. *P < 0.05, **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. ox-LDL group. (A color version of this figure is available in the online journal.)
Hydrogen inhibited the expression of adhesion molecules in ox-LDL-induced HUVECs
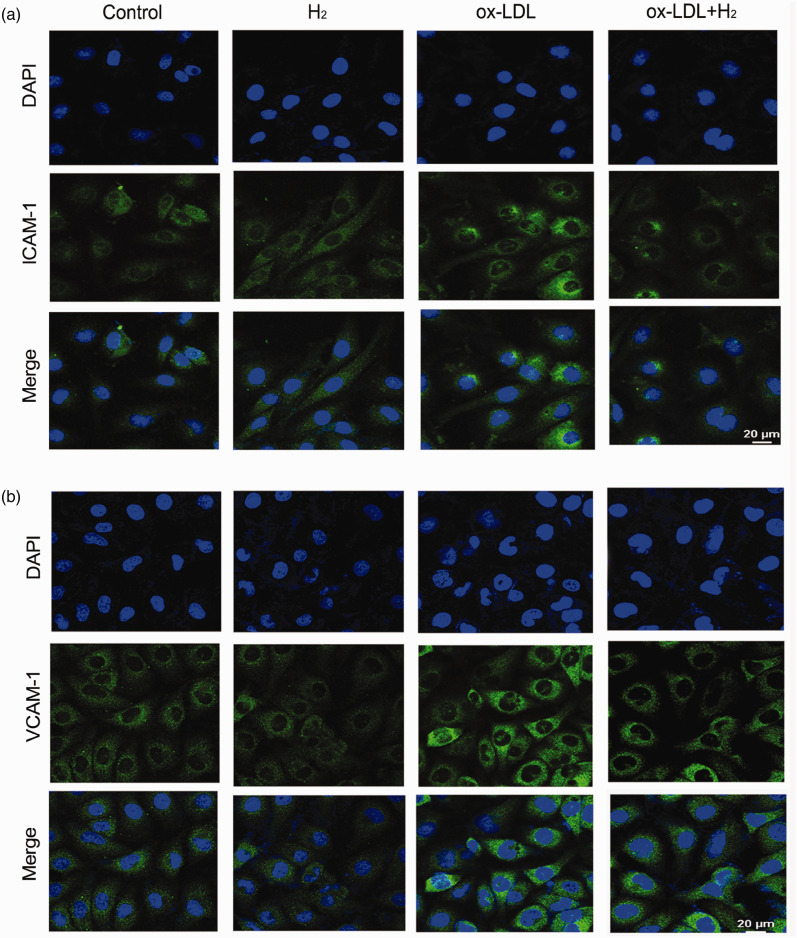
AS is a chronic inflammatory disease of arterial wall and adhesion molecules exert an important role in the process. 14 The expression of adhesion molecules increases after vascular endothelium damage, which mediates local adhesion and immigration of leukocytes. 15 In cell experiments, immunofluorescence analyses showed ox-LDL (100 μg/mL, 24 h) increased the expressions of ICAM-1 and VCAM-1 in HUVECs. However, hydrogen reversed the event (Figure 2).
Figure 2.
Hydrogen inhibited the protein expressions of ICAM-1 and VCAM-1 induced by ox-LDL in HUVECs. (a) Visualized ICAM-1 level by immunofluorescence experiment; (b) visualized VCAM-1 level by immunofluorescence experiment. (A color version of this figure is available in the online journal.)
Hydrogen down-regulated LOX-1/NF-κB signaling pathway in ox-LDL-induced HUVECs
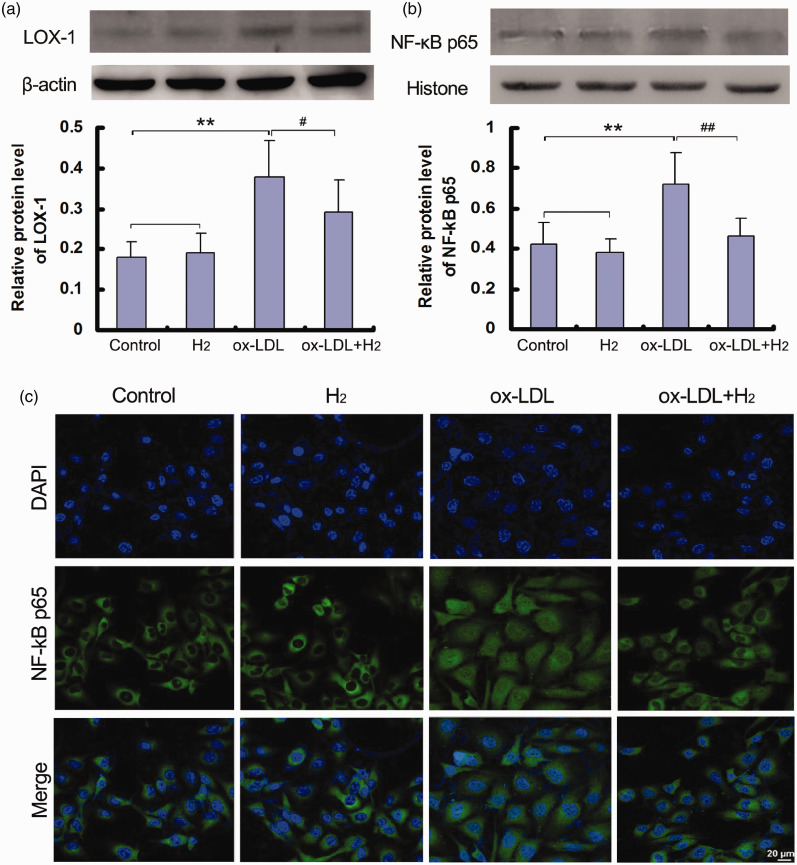
LOX-1 is the major receptor for ox-LDL in endothelial cells. 16 After combing with its ligands, ox-LDL results in endothelial oxidative injuries by modulating LOX-1-mediated oxidase signaling pathway. 17 More importantly, it is reported that LOX-1 activates NF-κB signaling pathway,18–19 which facilitates nuclear translocation and subsequently the gene expression of adhesion molecules. 20 In this study, incubation of HUVECs with ox-LDL up-regulated LOX-1 expression and NF-κB activity, while hydrogen treatment obviously reduced LOX-1 level and blocked translocation of NF-κB p65 into nucleus (Figure 3).
Figure 3.
Hydrogen down-regulated LOX-1/NF-κB signaling pathway in ox-LDL-induced HUVECs. The protein expressions of LOX-1 in cells and NF-κB p65 in nucleus were analyzed by Western blot or immunofluorescence. (a) LOX-1 protein level in cells by Western blot; (b) NF-κB p65 protein level in nucleus by Western blot; (c) visualized NF-κB p65 in nucleus by immunofluorescence experiment. Data were presented as mean ± SD of at least three independent experiments. **P < 0.01 vs. control group; #P < 0.05, ##P < 0.01 vs. ox-LDL group. (A color version of this figure is available in the online journal.)
Clinical data
Demographic characteristics of the study subjects with UA
Demographic characteristics of the study participants are reported in Table 1. Comparisons showed no statistical difference in gender proportion, age, stent placement, smoking habit, and drinking habit between control group and HRW group.
Table 1.
Characteristics of patients with UA treated with or without HRW.
| Control group | HRW group | |||
|---|---|---|---|---|
| Variables | n (%) | n (%) | P value | |
| Gender | ||||
| Male | 10 (50) | 14 (70) | 0.197 | |
| Female | 10 (50) | 6 (30) | ||
| Age | ||||
| ≤49 | 3 (15) | 4 (20) | 0.465 | |
| 50–59 | 11 (55) | 12 (60) | ||
| ≥60 | 6 (30) | 4 (20) | ||
| Stent placement | ||||
| Yes | 11 (55) | 14 (70) | 0.327 | |
| No | 9 (45) | 6 (30) | ||
| Smoking | ||||
| Yes | 4 (20) | 5 (25) | 1.000 | |
| No | 16 (80) | 15 (75) | ||
| Drinking | ||||
| Yes | 7 (35) | 8 (40) | 0.744 | |
| No | 13 (65) | 12 (60) |
UA: unstable angina; HRW: hydrogen-rich water.
Effect of HRW addition on angina symptoms in clinic
In this experiment, we analyzed the improvement of angina symptoms in the two groups after treatment. As shown in Table 2, among 20 cases in control group, 2 (10%) were markedly effective, 10 (50%) effective, and 8 (40%) ineffective; the total efficacy rate was 60%. Among 20 cases in HRW group, 4 (20%) were markedly effective, 14 (70%) effective, and 2 (10%) ineffective; the total efficacy rate was 90%. The data showed HRW plus conventional drugs had a better effect on relieving symptoms of angina (60% vs. 90%, χ2 = 4.800, P < 0.05).
Table 2.
Comparison of angina symptom reduction of UA patients after treatment for three months.
| Group | Case | Markedly effective n (%) | Effective n (%) | Ineffective n (%) | Total effective n (%) |
|---|---|---|---|---|---|
| Control group | 20 | 2 (10) | 10 (50) | 8 (40) | 12 (60) |
| HRW group | 20 | 4 (20) | 14 (70) | 2 (10) | 18 (90) |
Note: χ2 = 4.800, P < 0.05 (P = 0.028).
UA: unstable angina; HRW: hydrogen-rich water.
Effect of HRW addition on serum lipid and lipoprotein profiles of UA participants
The serum lipid and lipoprotein levels in each individual patient with UA are presented in Table 3. Clinical biochemical testing showed that the effective rates to lower serum TC, LDL-C, and apoB levels in HRW group were increased compared with those in control group (TC, 35% vs. 15%; LDL-C, 40% vs. 20%; apoB, 40% vs. 15%). However, the serum HDL-C and apoA1 levels were not significantly altered by HRW addition which is not shown in this paper.
Table 3.
Effects of HRW addition on serum lipid and lipoprotein levels in each individual patient with UA.
|
TC |
LDL-C |
apoB |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Control group |
HRW group |
Control group |
HRW group |
Control group |
HRW group |
|||||||
| Patient number | Before treatment (mM) | After treatment (mM) | Before treatment (mM) | After treatment (mM) | Before treatment (mM) | After treatment (mM) | Before treatment (mM) | After treatment (mM) | Before treatment (g/L) | After treatment (g/L) | Before treatment (g/L) | After treatment (g/L) |
| 1 | 5.99a | 4.46 | 6.41a | 4.51 | 4.38a | 2.19 | 3.80a | 2.51 | 1.17 | 0.99 | 1.40a | 0.84 |
| 2 | 5.64a | 4.19 | 6.09a | 3.62 | 2.22 | 2.58 | 3.89a | 1.92 | 0.70 | 0.88 | 1.26a | 0.65 |
| 3 | 5.37 | 5.08 | 5.25 | 5.07 | 3.87 | 3.70 | 3.14 | 2.83 | 1.18a | 0.73 | 1.09 | 0.94 |
| 4 | 5.06 | 4.60 | 4.82 | 4.43 | 2.95 | 2.50 | 2.68 | 2.34 | 0.97 | 0.87 | 0.93 | 0.86 |
| 5 | 4.90 | 5.14 | 4.73a | 3.02 | 2.48a | 1.74 | 2.81a | 1.49 | 0.85 | 0.69 | 1.05a | 0.61 |
| 6 | 4.73a | 3.60 | 4.61 | 4.29 | 2.57a | 1.89 | 2.46 | 2.37 | 1.04a | 0.75 | 0.84 | 0.86 |
| 7 | 4.62 | 4.35 | 4.35 | 5.95 | 2.42 | 2.48 | 2.48 | 3.18 | 0.96 | 0.88 | 0.99 | 1.04 |
| 8 | 4.61 | 3.76 | 4.25a | 3.10 | 2.61 | 2.54 | 2.42a | 1.39 | 0.92 | 0.88 | 0.79a | 0.55 |
| 9 | 4.40 | 4.68 | 4.21 | 4.25 | 2.15 | 1.80 | 1.95 | 2.18 | 0.73 | 0.63 | 0.73 | 0.85 |
| 10 | 4.39 | 4.45 | 4.17 | 4.01 | 2.94 | 2.62 | 2.51 | 2.42 | 1.07 | 0.93 | 0.87 | 1.05 |
| 11 | 4.38 | 4.33 | 4.04a | 2.32 | 2.86 | 3.06 | 2.31a | 0.98 | 0.98 | 1.04 | 0.73a | 0.46 |
| 12 | 4.04 | 3.31 | 4.03 | 4.07 | 2.31 | 1.91 | 1.96 | 1.82 | 0.76 | 0.65 | 0.75 | 0.74 |
| 13 | 4.04 | 3.77 | 4.00a | 2.96 | 2.91 | 3.10 | 2.14a | 1.29 | 0.98 | 1.07 | 0.70a | 0.51 |
| 14 | 3.89 | 3.46 | 3.90a | 3.04 | 1.80 | 1.57 | 1.73a | 0.95 | 0.66 | 0.60 | 0.60a | 0.37 |
| 15 | 3.81 | 3.07 | 3.87 | 4.45 | 2.02a | 1.49 | 2.00 | 3.36 | 0.80a | 0.62 | 0.74 | 0.73 |
| 16 | 3.67 | 3.02 | 3.64 | 3.34 | 1.65 | 1.48 | 1.86 | 1.75 | 0.64 | 0.59 | 0.72 | 0.67 |
| 17 | 3.48 | 3.87 | 3.26 | 2.68 | 1.47 | 1.82 | 1.46a | 1.13 | 0.58 | 0.66 | 0.60a | 0.45 |
| 18 | 3.32 | 3.43 | 3.11 | 5.29 | 1.66 | 1.46 | 1.46 | 3.09 | 0.66 | 0.59 | 0.60 | 0.96 |
| 19 | 3.05 | 4.54 | 2.76 | 4.54 | 1.19 | 2.05 | 1.50 | 2.79 | 0.81 | 0.71 | 0.60 | 0.92 |
| 20 | 2.91 | 3.87 | 2.74 | 2.49 | 1.73 | 2.19 | 1.31 | 1.59 | 0.65 | 0.73 | 0.58 | 0.56 |
| Mean | 4.32 | 4.05 | 4.21 | 3.87 | 2.41 | 2.21 | 2.29 | 2.07 | 0.86 | 0.77 | 0.83 | 0.73 |
| SD | 0.83 | 0.63 | 0.95 | 0.99 | 0.79 | 0.61 | 0.72 | 0.75 | 0.18 | 0.16 | 0.23 | 0.21 |
| Effectiverate | 15% | 35% | 20% | 40% | 15% | 40% | ||||||
aPatients whose lipid or lipoprotein level was reduced above 20% after treatment for three months compared with the baseline data before treatment. The effective rate was calculated by the marked patients’ number to total patients’ number.
HRW: hydrogen-rich water, UA: unstable angina, TC: total cholesterol, LDL-C: low-density lipoprotein-cholesterol, apoB: apolipoprotein B.
Discussion
CHD usually results from the occlusion of coronary artery by atherosclerotic plaque. When the occlusion >75%, the increase of physical activity can cause the relative insufficiency of coronary blood supply and mild angina pectoris, which is called “stable angina”. However, once the atherosclerotic plaque ruptures suddenly and forms thrombus on the surface, it will aggravate the degree of stenosis, and then cause UA. UA can further develop into acute myocardial infarction, or even sudden death. UA is an acute and critical illness with characteristics of high hospitalization rate, high disability rate, and high mortality. 21 Even though conventional medicine benefits the patients of UA, various drug resistance and side effects raise the difficulty of the treatment. Therefore, better strategies for delaying the development of atherosclerosis and treating UA have been seeking.
It has been well established that oxidative stress and inflammation play a key role in the initiation and progression of atherothrombotic disease. 22 A variety of cardiovascular risk factors, such as hypertension, hyperlipidemia, diabetes mellitus or smoking, can stimulate ROS generation in vascular wall. 23 ROS can peroxidize lipid components and form ox-LDL, which deposits under the endothelium and promote plaque enlargement. 24 Furthermore, ox-LDL can regulate redox-sensitive transcription factors such as NF-κB and accelerates the synthesis of adhesion molecules and inflammatory mediators in endothelial cells and macrophages. 25 These cytokines can affect the stability of plaque and induce platelet activation. 26 Based on the above, it can be assumed that anti-oxidation and anti-inflammation are also important targets for clinical treatment of UA. Molecular hydrogen, a potent antioxidative and anti-inflammatory agent, has been explored as an ideal therapy tool for many diseases over the last 10 years. Compared with other antioxidants, hydrogen has the following advantages: (1) It can be directly dispersed into cells, even organelles because of small molecular weight and strong penetration; (2) So far, any adverse reaction has not been found after drinking or inhaling hydrogen; (3) It is convenient to use hydrogen production cup.
In order to elucidate protective effect of hydrogen on UA, we constructed an in vitro AS model by exposing endothelial cells to ox-LDL. Effects of hydrogen on ox-LDL-induced oxidative stress and inflammation in HUVECs were investigated. Our data indicated that hydrogen treatment inhibited mitochondrial ROS generation and decreased MDA level in ox-LDL-stimulated HUVECs. Meanwhile, hydrogen down-regulated the expressions of adhesion molecules, including both ICAM-1 and VCAM-1, in HUVEC cells. Ox-LDL cannot be uptaken by LDL receptor, instead, it is mainly recognized by LOX-1 in endothelial cells, macrophages, and smooth muscle cells. 27 The uptake of ox-LDL can sharply increase intracellular ROS such as superoxide (O2•) and hydrogen peroxide. In turn, ROS promotes the expression of LOX-1, thereby resulting in further ROS generation. 28 As a consequence, the excessive ROS triggers the activation of NF-κB 29 and increases expression of adhesion molecules and proinflammatory cytokines via LOX-1/NF-κB signaling pathway. 30 Our results indicated that hydrogen treatment obviously down-regulated LOX-1/NF-kB signaling pathway in ox-LDL-induced HUVECs (Figure 3).
In this study, we also evaluated the effect of HRW supplement on UA in clinic and the results claimed HRW combined with conventional drugs had a better effect on relieving angina symptoms than conventional drugs alone. Our clinical data analysis also indicated that hydrogen could regulate metabolism and reduce blood lipid. Serum lipid testing showed HRW as a complementary medicine could more effectively lower UA patients’ TC, LDL-C, and apoB levels compared with conventional treatment. These data were consistent with those of our previous research for metabolic syndrome patients. 3 Furthermore, the clinical follow-up also showed that angina pectoris, sleep, vitality, and mood of the patients in HRW group were improved compared with control group. The times of return visit in HRW group were less than those of control group. No obvious adverse reaction was found during the intervention except one case in control group reporting of stomach discomfort.
In conclusion, the present study demonstrates that hydrogen not only inhibits oxidative stress and inflammation by suppressing LOX-1/NF-kB signaling pathway in endothelial cells but also improves serum cholesterol level of UA patients in clinic. These effects contribute to the easement of angina symptoms in UA. Our study suggests hydrogen can be a potential strategy for the treatment and prevention of atherosclerotic cardiovascular disease in the future. Of course, considering the small sample of participants in this study, an appropriately designed, large-scale, prospective clinical study is therefore necessary to confirm our findings.
Footnotes
AUTHORS’ CONTRIBUTIONS: All authors participated in the design and implementation process of the study. HT analyzed data and YS wrote the manuscript. All authors approved the final version of the manuscript.
DECLARATION OF CONFLICTING INTERESTS: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ETHICAL APPROVAL: The study was approved by the ethics committee of Shandong First Medical University and Chinese Clinical Trial Registry (ChiCTR2100042722). Written informed consent was obtained from every participant prior to participating in this study.
FUNDING: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the financial supports from Natural Science Foundations of China (81770855, 81600681, 81800394), Academic Promotion Programme of Shandong First Medical University (2019QL010, 2019PT009) and Key Research and Development project of Shandong Province (No. 2019gsf108260).
ORCID iD: Yanhong Si https://orcid.org/0000-0002-2318-8080
References
- 1.World Health Organization. Cardiovascular diseases (CVDs). Fact sheet N.317.
- 2.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 investigators. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med 2004; 350: 1495–504 [DOI] [PubMed] [Google Scholar]
- 3.Ross R. Atherosclerosis: an inflammatory disease. N Engl J Med 1999; 340:115–26 [DOI] [PubMed] [Google Scholar]
- 4.Falk E, Nakano M, Bentzon JF, Finn AV, Virmani R. Update on acute coronary syndromes: the pathologists’ view. Eur Heart J 2013; 34:719–28 [DOI] [PubMed] [Google Scholar]
- 5.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. Pravastatin or atorvastatin evaluation and infection therapy-thrombolysis in myocardial infarction 22 (PROVE IT-TIMI 22) investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med 2005; 352:20–8 [DOI] [PubMed] [Google Scholar]
- 6.Bai G, Li H, Ge Y, Zhang Q, Zhang J, Chen M, Liu T, Wang H. Influence of hydrogen-rich saline on hepatocyte autophagy during laparoscopic liver ischaemia-reperfusion combined resection injury in miniature pigs. J Vet Res 2018; 62:395–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qian L, Shen J, Chuai Y, Cai J. Hydrogen as a new class of radioprotective agent. Int J Biol Sci 2013; 9:887–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shao A, Wu H, Hong Y, Tu S, Sun X, Wu Q, Zhao Q, Zhang J, Sheng J. Hydrogen-rich saline attenuated subarachnoid hemorrhage-induced early brain injury in rats by suppressing inflammatory response: possible involvement of NF-κB pathway and NLRP3 inflammasome. Mol Neurobiol 2016; 53:3462–76 [DOI] [PubMed] [Google Scholar]
- 9.Song G, Tian H, Qin S, Sun X, Yao S, Zong C, Luo Y, Liu J, Yu Y, Sang H, Wang X. Hydrogen decreases athero-susceptibility in apolipoprotein B-containing lipoproteins and aorta of apolipoprotein E knockout mice. Atherosclerosis 2012; 221:55–65 [DOI] [PubMed] [Google Scholar]
- 10.Song G, Li M, Sang H, Zhang L, Li X, Yao S, Yu Y, Zong C, Xue Y, Qin S. Hydrogen-rich water decreases serum LDL-cholesterol levels and improves HDL function in patients with potential metabolic syndrome. J Lipid Res 2013; 54:1884–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fridovich I. Superoxide radical: an endogenous toxicant. Annu Rev Pharmacol Toxicol 1983; 23:239–57 [DOI] [PubMed] [Google Scholar]
- 12.Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE, Jr, Chavey WE, 2nd Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Smith SC Jr; WRITING GROUP MEMBERS; ACCF/AHA TASK FORCE MEMBERS. 2011 ACCF/AHA focused update incorporated into the ACC/AHA 2007 guidelines for the management of patients with unstable angina/Non-ST-Elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2011; 123:e426–579 2011 [DOI] [PubMed] [Google Scholar]
- 13.Ministry of health of the People’s Republic of China. Clinical research guidelines for new traditional Chinese drug. Beijing: Drug Administration Bureau of Ministry of health, 1993
- 14.Libby P. Mechanisms of acute coronary syndromes and their implications for therapy. N Engl J Med 2013; 368:2004–13 [DOI] [PubMed] [Google Scholar]
- 15.Buffon A, Biasucci LM, Liuzzo G, D'Onofrio G, Crea F, Maseri A. Wide spread coronary inflammation in unstable angina. N Engl J Med 2002; 347:5–12 [DOI] [PubMed] [Google Scholar]
- 16.Pothineni NVK, Karathanasis SK, Ding Z, Arulandu A, Varughese KI, Mehta JL. LOX-1 in atherosclerosis and myocardial ischemia: biology, genetics, and modulation. J Am Coll Cardiol 2017; 69:2759–68 [DOI] [PubMed] [Google Scholar]
- 17.Tsai KL, Chen LH, Chiou SH, Chiou GY, Chen YC, Chou HY, Chen LK, Chen HY, Chiu TH, Tsai CS, Ou HC, Kao CL. Coenzyme Q10 suppresses oxLDL-induced endothelial oxidative injuries by the modulation of LOX-1-mediated ROS generation via the AMPK/PKC/NADPH oxidase signaling pathway. Mol Nutr Food Res 2011; 55:S227–40 [DOI] [PubMed] [Google Scholar]
- 18.Balzan S, Lubrano V. LOX-1 receptor: a potential link in atherosclerosis and cancer. Life Sci 2018; 198:79–86 [DOI] [PubMed] [Google Scholar]
- 19.Xu Y, Ruan S, Xie H, Lin J. Role of LOX-1 in ang II-induced oxidative functional damage in renal tubular epithelial cells. Int J Mol Med 2010; 26:679–90 [DOI] [PubMed] [Google Scholar]
- 20.Chen XP, Zhang TT, Du GH. Lectin-like oxidized low-density lipoprotein receptor-1, a new promising target for the therapy of atherosclerosis? Cardiovasc Drug Rev 2007; 25:146–61 [DOI] [PubMed] [Google Scholar]
- 21.Mockel M, Searle J, Hamm C, Slagman A, Blankenberg S, Huber K, Katus H, Liebetrau C, Muller C, Muller R, Peitsmeyer P, von Recum J, Tajsic M, Vollert JO, Giannitsis E. Early discharge using single cardiac troponin and copeptin testing in patients with suspected acute coronary syndrome (ACS): a randomized, controlled clinical process study. Eur Heart J 2015; 36:369–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tousoulis D, Briasoulis A, Papageorgiou N, Tsioufis C, Tsiamis E, Toutouzas K, Stefanadis C. Oxidative stress and endothelial function: therapeutic interventions. Recent Pat Cardiovasc Drug Discov 2011; 6:103–14 [DOI] [PubMed] [Google Scholar]
- 23.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res 2000; 87:840–4 [DOI] [PubMed] [Google Scholar]
- 24.Bonomini F, Tengattini S, Fabiano A, Bianchi R, Rezzani R. Atherosclerosis and oxidative stress. Histol Histopathol 2008; 23:381–90 [DOI] [PubMed] [Google Scholar]
- 25.Brasier AR. The nuclear factor-kappaB-interleukin-6 signalling pathway mediating vascular inflammation. Cardiovasc Res 2010; 86:211–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mozzini C, Garbin U, Stranieri C, Salandini G, Pesce G, Fratta Pasini AM, Cominacini L. Nuclear factor kappa B in patients with a history of unstable angina: case re-opened. Intern Emerg Med 2018; 13:699–707 [DOI] [PubMed] [Google Scholar]
- 27.Kume N, Kita T. Lectin-like oxidized low-density lipoprotein receptor- 1 (LOX-1) in atherogenesis. Trends Cardiovasc Med 2001; 11:22–7 [DOI] [PubMed] [Google Scholar]
- 28.Chen XP, Xun KL, Wu Q, Zhang TT, Shi JS, Du GH. Oxidized low density lipoprotein receptor-1 mediates oxidized low density lipoproteininduced apoptosis in human umbilical vein endothelial cells: role of reactive oxygen species. Vascul Pharmacol 2007; 47:1–9 [DOI] [PubMed] [Google Scholar]
- 29.Bao MH, Zhang YW, Zhou HH. Paeonol suppresses oxidized low density lipoprotein induced endothelial cell apoptosis via activation of LOX-1/p38MAPK/NF-kappaB pathway. J Ethnopharmacol 2013; 146:543–51 [DOI] [PubMed] [Google Scholar]
- 30.Xu S, Ogura S, Chen J, Little PJ, Moss J, Liu P. LOX-1 in atherosclerosis: biological functions and pharmacological modifiers. Cell Mol Life Sci 2013; 70:2859–72 [DOI] [PMC free article] [PubMed] [Google Scholar]