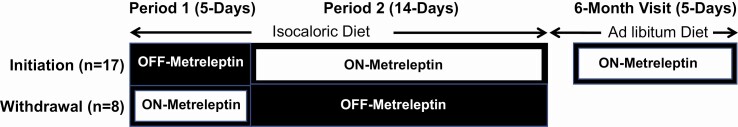
Figure 1.
Study design schematic, nonrandomized, crossover study. Initiation patients (n = 17) were studied for the first 5 days without metreleptin (Period 1) and then treated with metreleptin for the next 14 days (Period 2). Withdrawal patients (n = 8) were studied for the first 5 days on their home dose of metreleptin (Period 1) and then withdrawn from metreleptin for the next 14 days (Period 2). Patients in the initiation cohort continued self-administered metreleptin treatment after discharge and were reevaluated after 6 months of metreleptin treatment on an ad libitum diet.