FIG 10.
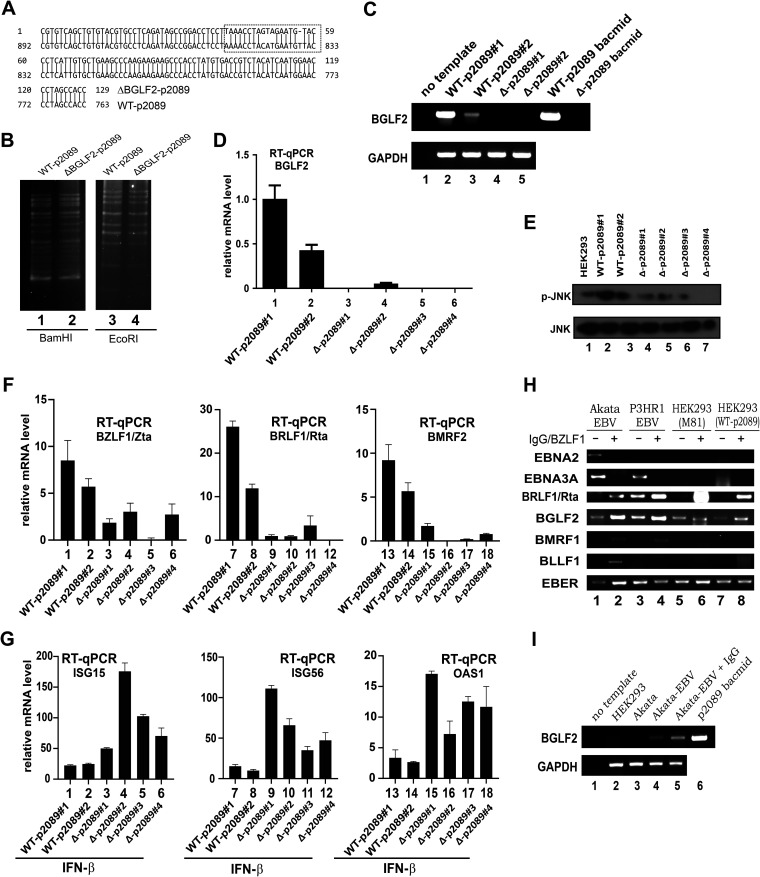
BGLF2 suppresses IFN-β signaling in infected cells. (A) Verification of BGLF2-deficient mutant by sequencing. An alignment of mutant and wild-type sequence is shown. (B) Verification of genome integrity by BamHI and EcoRI patterning. WT-p2089 and ΔBGLF2-p2089 bacmids were digested with BamHI or EcoRI at 37°C for 24 h. The digested products were run on 2% agarose gel overnight at a low voltage. (C and D) Verification of BGLF2 mRNA expression by RT-PCR or RT-qPCR. The primers used can preferentially amplify WT-BGLF2 mRNA but not that of ΔBGLF2. A TaqMan probe was also used in the RT-qPCR. (E) Verification of BGLF2 deficiency through Western blot analysis of the p-JNK levels. (F) Expression of immediate-early and late lytic genes in ΔBGLF2-p2089 stable cells. Cell clones were treated with DMSO or 400 μM TPA plus 5 mM NaBt for 48 h. (G) ISG induction in ΔBGLF2-p2089 stable cells. Cell clones were treated with 1,000 U/ml of IFN-β. ISG15, ISG56 and OAS1 transcripts were analyzed by RT-qPCR. (H) Expression profiles of latent and lytic genes in HEK293 cells stably carrying WT-p2089 and other EBV-positive cells. Lytic reaction of Akata cells was induced by IgG cross-linking. A lytic reactivation of other cells was induced by transfection with a BZLF1/Zta expression plasmid. (I) Expression of BGLF2 gene in EBV-positive cell lines. EBV-negative and -positive Akata cells were induced for EBV lytic cycle using IgG and the expression of BGLF2 mRNA was analyzed by RT-PCR. HEK293 cells and p2089 bacmid were used as negative and positive controls, respectively.