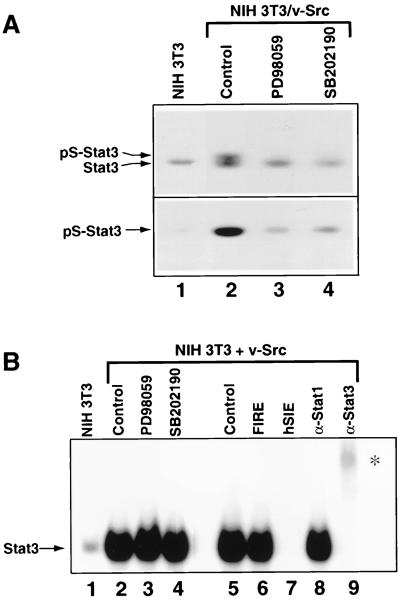
FIG. 5.
Analyses of constitutive Stat3 serine phosphorylation and SIE-binding activity induced by v-Src. (A) Western blot analysis of whole-cell lysates prepared from normal NIH 3T3 fibroblasts and Src-transformed counterparts treated with PD98059 or SB202190 for 6 h or left untreated (lanes 2 to 4). Samples were probed with antibodies specific to phosphoserine-727 (bottom) or the N-terminal portion (top) of Stat3. (B) Nuclear extracts were prepared from NIH 3T3 cells transfected with v-Src. Equal amounts of total protein were incubated with 32P-labeled M67SIE and subjected to EMSA. Cells were transfected with empty vector alone (NIH 3T3) or v-Src vector and treated with PD98059 or SB202190 for 6 h or left untreated (lanes 2 to 5). Competitions of radiolabeled M67SIE-binding activity present in nuclear extracts of NIH 3T3 cells transfected with v-Src alone (lanes 6 and 7) were performed with a 100-fold molar excess of unlabeled M67SIE or the unrelated FIRE oligonucleotides. Supershifts (lanes 8 and 9) were performed with antibodies specifically recognizing either Stat1 or Stat3 (α-Stat1, α-Stat3). The asterisk indicates positions of supershifted complexes.