Figure 3. Regulation of Mitochondria–Lysosome Contact Tethering/Untethering by Rab7 GTP Hydrolysis.
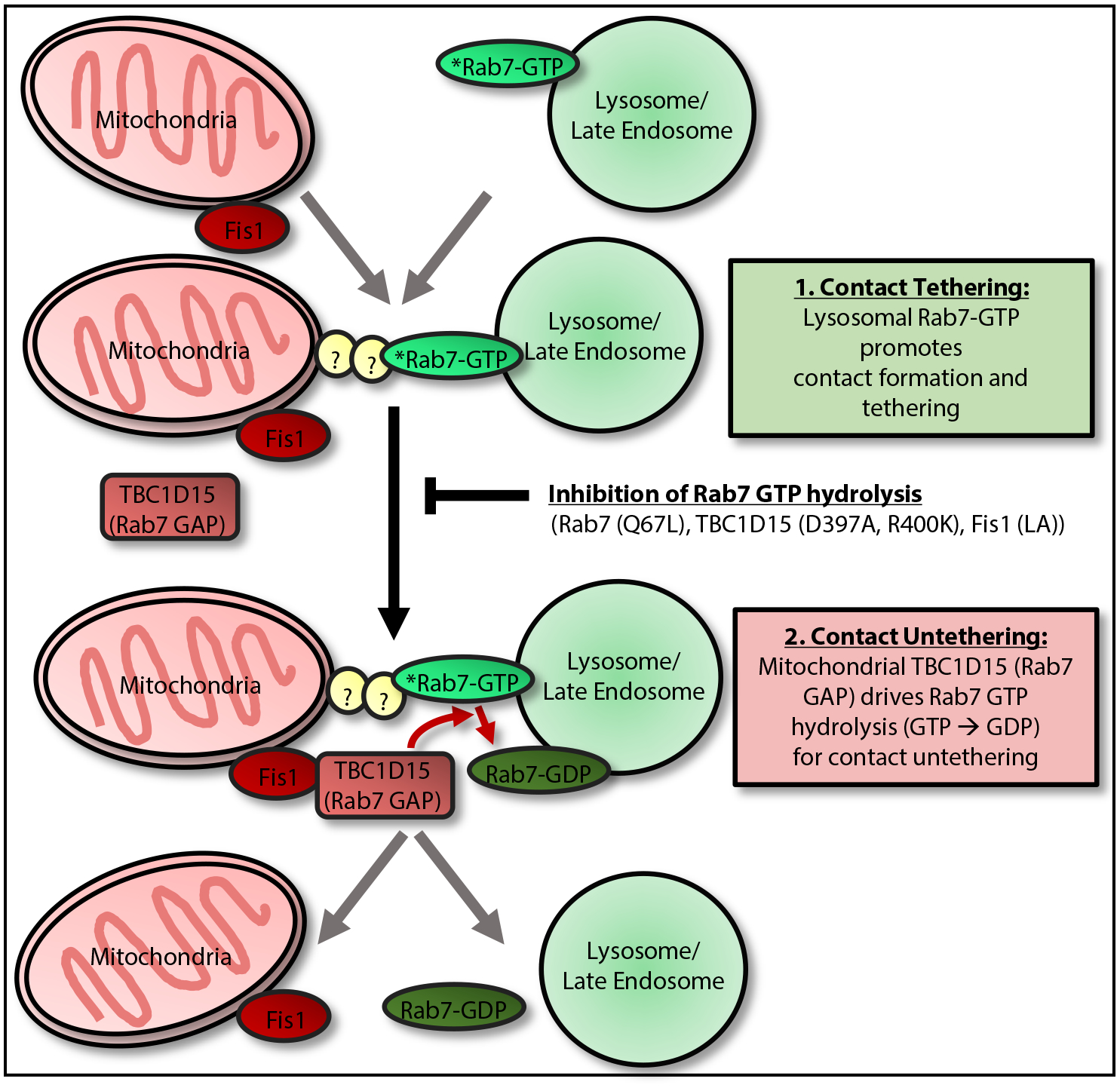
Mitochondria–lysosome contact dynamics involve: (1) contact tethering, which is promoted by lysosomal GTP-bound Rab7 and is potentially mediated by Rab7 effector proteins (which bind GTP-bound Rab7) to directly tether lysosomes to mitochondria [53]; (2) contacts subsequently undergo untethering, which is mediated by recruitment of cytosolic TBC1D15 (Rab7 GAP) to mitochondria via the outer mitochondrial membrane protein Fis1 [61–63]. At mitochondria–lysosome contact sites, mitochondrial TBC1D15 is able to interact with lysosomal GTP-bound Rab7 to drive Rab7 GTP hydrolysis from a GTP-bound to GDP-bound state. GDP-bound Rab7 can no longer bind Rab7 effectors and also loses its lysosomal membrane localization, leading to the loss of tethers and mitochondria–lysosome contact untethering [53]. Inhibition of Rab7 GTP hydrolysis with either mutant GTP-bound Rab7 (Q67L), which is unable to undergo GTP hydrolysis, mutant TBC1D15 (D397A or R400K), which lacks GAP activity [61], or mutant Fis1 (LA), which is unable to recruit TBC1D15 to mitochondria [61], all prevent efficient mitochondria–lysosome contact untethering, resulting in prolonged contacts [53]. Additional proteins apart from Rab7 may also be involved in regulating mitochondria–lysosome contact tethering.
