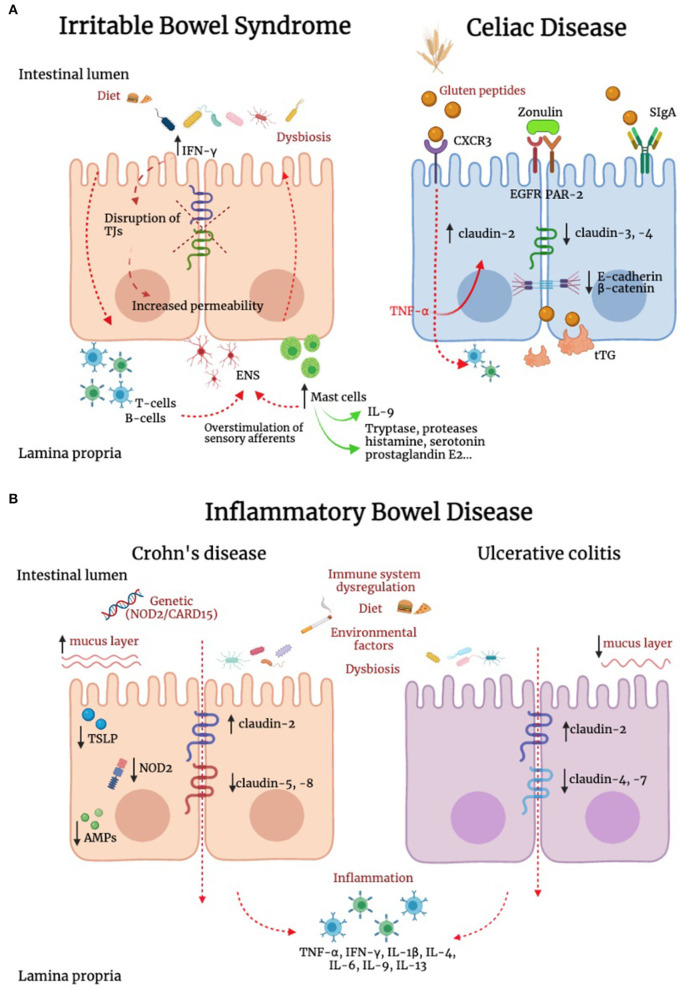
Figure 2.
Alteration of IEB in irritable bowel syndrome, inflammatory bowel disease and celiac disease. (A) In irritable bowel syndrome (IBS), tryptase, histamine, and interferon-γ (IFN-γ) are increased and can contribute to TJ disruption. In addition, IL-9 is produced by innate lymphoid cells, T helper cells and mast cells. This latter cell population has a key role in IBS pathophysiology, and can modify TJ protein expression. In addition, immune mediators, including histamine, serotonin and proteases evoke sensory afferent over-stimulation and contribute to symptom generation. In celiac disease (CD), intestinal epithelial cells recognize gluten peptides through CXCR3, which increases IEB permeability through zonulin release and its transactivation of epidermal growth factor receptor (EGFR) and protease activated receptor 2 (PAR-2). Transcytosis of gluten peptides occurs after peptide recognition by secretory immunoglobulin A (SIgA). A reduction in the expression of E-cadherin and β-catenin was found in intestinal biopsies of CD patients. Active tissue transglutaminase2 (tTG2) deamidates gluten peptides, contributing to the development of a cell mediated pro-inflammatory immune response. (B) Genetics, environment, diet, immune system dysregulation and dysbiosis represent some of the complex mechanisms responsible for inflammatory bowel diseases (IBD). Inflammation down-regulates TJs proteins contributing to IEB alteration. In IBD patients has been reported an upregulation of the pore-forming claudin-2 and downregulation of occludin. Channel-forming claudins are up-regulated by cytokines including TNF-α and IL-13, leading to an increased permeability for ions and water. Occludin is down-regulated by inflammatory processes (e.g., TNF-α and IFN-γ), leading to increased paracellular permeability for macromolecules. Moreover, it has been shown a downregulation of claudin-5 and -8 in Crohn's disease and claudin-4 and -7 in Ulcerative Colitis (UC). In Crohn's disease, the stimulation of NOD2, a sensor of Gram-positive bacteria, induces the production of pro-inflammatory mediators, which concur to IEB dysfunction. Differently from UC, in Crohn's disease the mucus layer is thicker, suggesting an increase in MUC2 expression and goblet cells hyperplasia. In patients with Crohn's disease, intestinal epithelial cells (IECs) failed to produce thymic stromal lymphopoietin (TSLP), with consequent inability to control IL-12, produced by dendritic cells and involved in the development of Thelper 2 cells, resulting in alteration of intestinal homeostasis. Compared to UC, in which the antimicrobial peptides (AMPs) system seems to be adequately induced, Crohn's disease is characterized by lower levels of AMPs. Contrasting evidence are available on the role of smoking in UC and Crohn's disease.