Figure 10. Comparison of internal torsion angle dynamics between wild-type and CheY A113P.
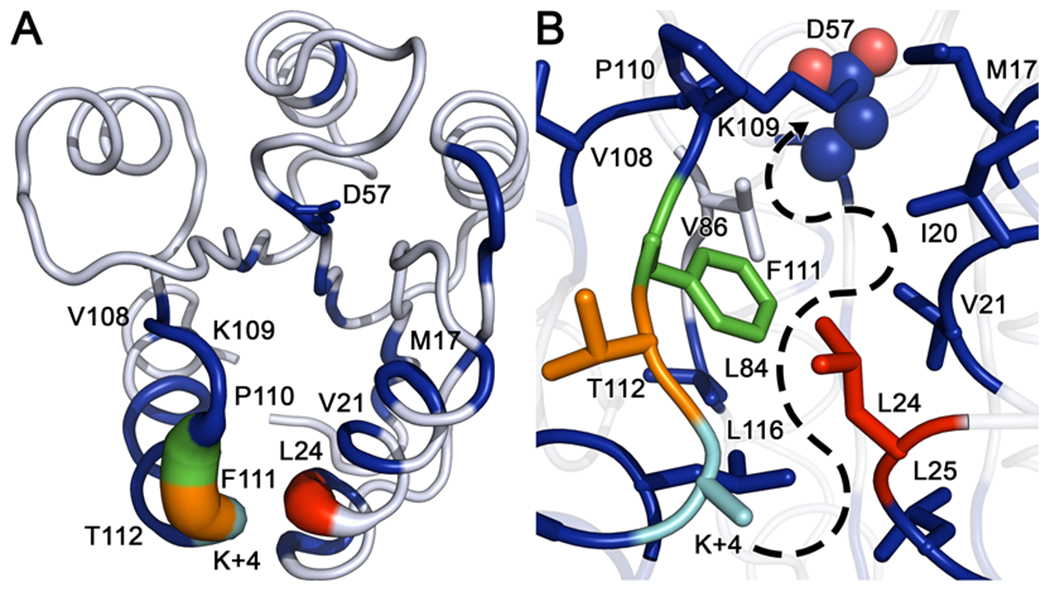
(A) Kullback-Leibler divergence between the two CheY variant ensembles mapped to the wild-type CheY crystal structure (PDB ID: 3CHY). Tube thickness and color warmth (blue to red) scale proportionally to amount of perturbation observed in dihedral angle distributions. Residues colored white failed to deviate above the thresholds set for statistical significance. (B) Closeup of the area between the β5α5 and α1 regions, showing densely packed hydrophobic cluster and putative path of signal transference from position K+4 to the active site (phosphorylatable D57 is shown as spheres). Significantly perturbed residues such as V21, L24, L84, F111 and L116 feature large hydrophobic side chains inserted into the cluster, while residues such as M17, V108, P110 and T112 are oriented away from the hydrophobic core. Residue color-coding matches panel A.
