Figure 11. Overlay of known CheY partner binding residues (red) and positions determined to be significantly perturbed by the A113P substitution (light blue).
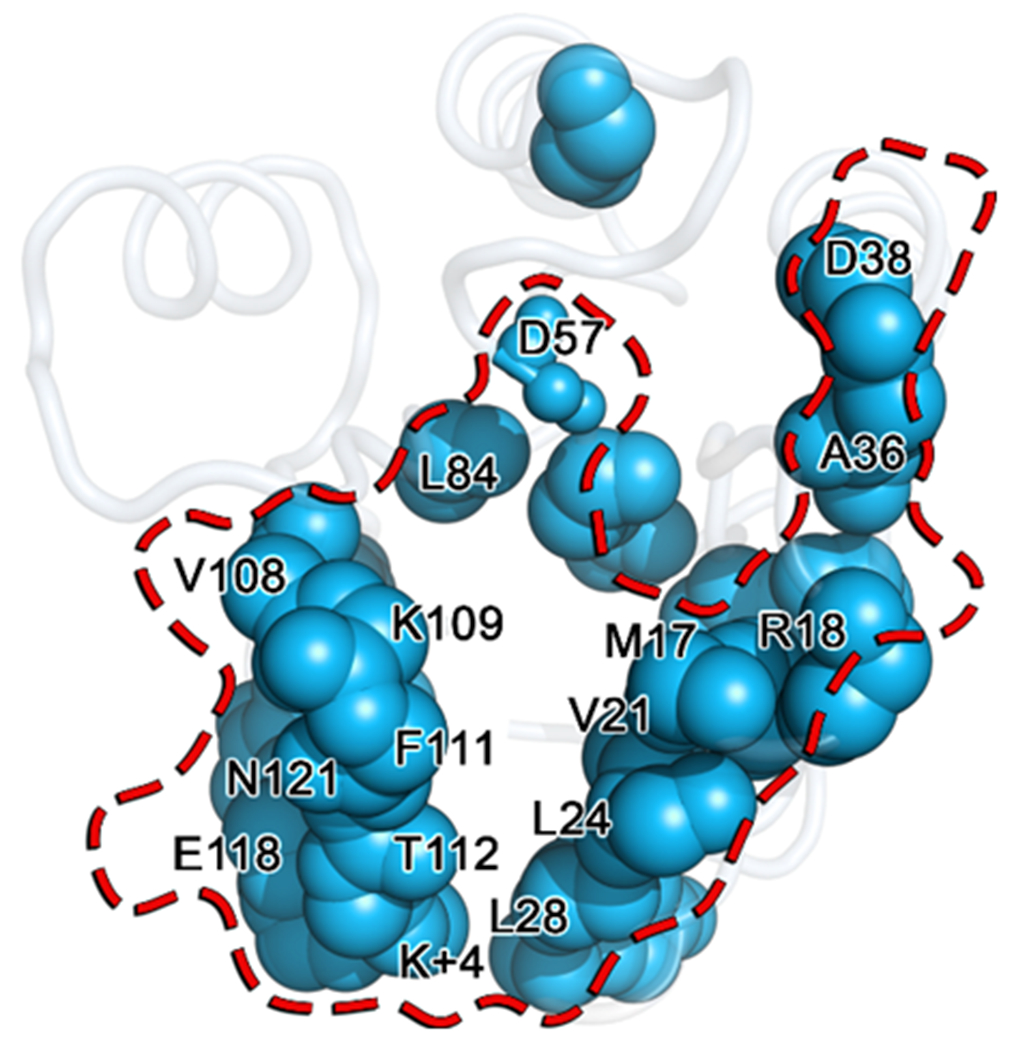
Residues identified as significantly perturbed by the A113P substitution using the KL divergence are shown as blue spheres mapped onto the wild-type CheY crystal structure (PDB ID: 3CHY). Residues known to be involved in CheY binding to its partners, CheAP1, CheZ and/or FliM1-16, are denoted with a transparent surface representation outlined in red. Residue positions shared by both groups are labeled, revealing a high degree of overlap.
