Extended Data Fig. 5. Dcp1/Dcp2ext conformational equilibria is important for substrate recognition, liquid-like behavior, and proper regulation of decapping in condensates.
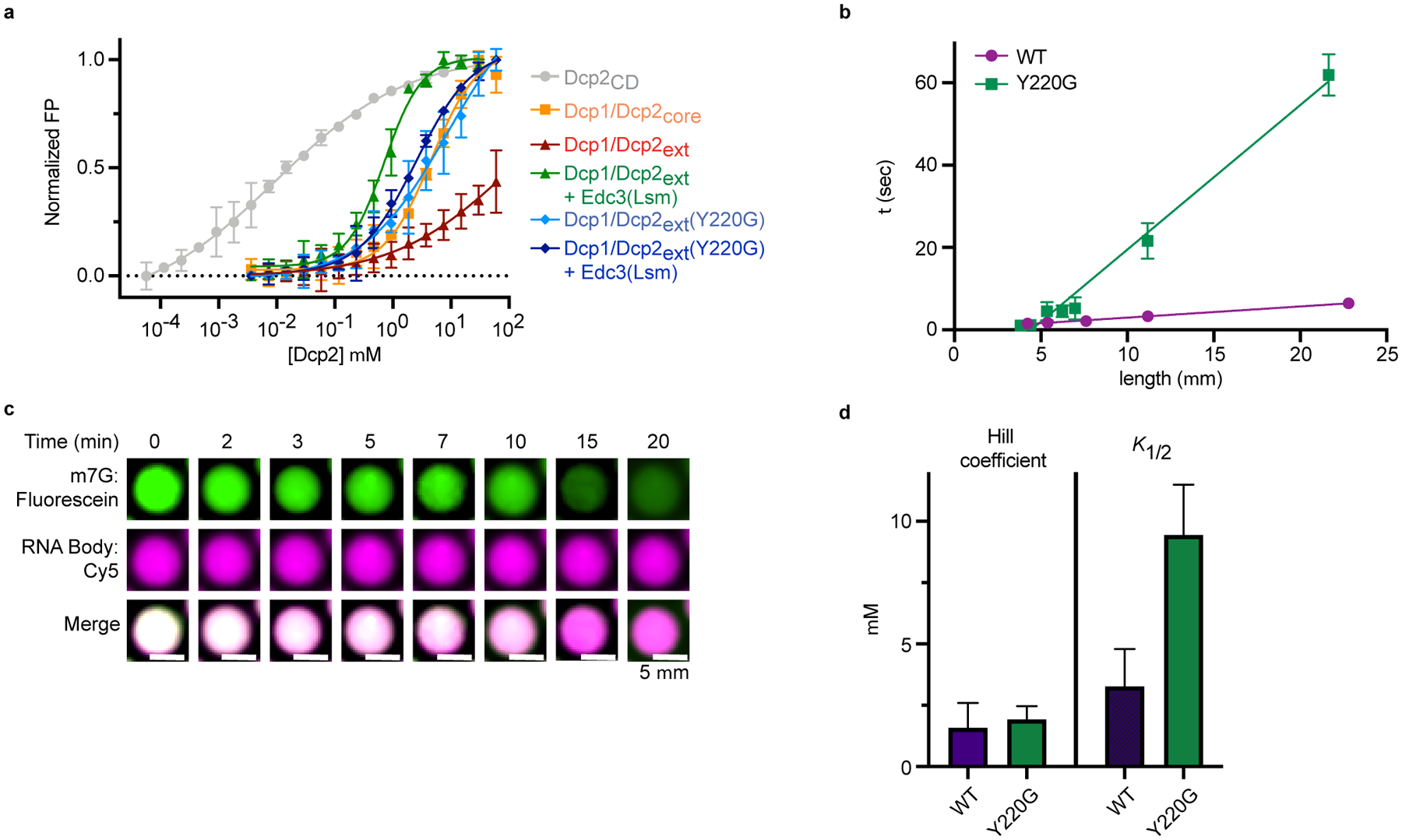
a, FP curves for various Dcp2 constructs binding to U30mer RNA. Data are normalized to the span between minimum and maximum mP values for each protein tested. Dcp1/Dcp2ext was normalized to span between its minimum and average maximum for all proteins tested. Data are presented as mean ± s.e.m. for three independent experiments and error bars are not show when smaller than the data point. b, Fusion of Dcp1/Dcp2ext(Y220G) condensates occurs slower than wild-type droplets of similar size. Time (τ) data presented are from fits of exponential decrease in droplet length following initial fusion event and error represents standard error of the fit and are not depicted when smaller than the data point. Representative micrographs are from three independent experiments with similar results. c, Decapping of dual-labeled RNA substrate by Dcp1/Dcp2ext(Y220G) by fluorescence microscopy. Representative micrographs are from twenty droplets collected over two independent experiments with similar results. d, The Y220G mutation does not affect the cooperativity of activation by Edc3 but increases the K1/2 of activation three-fold. Hill coefficients and K1/2 are presented as mean ± s.e.m. for experimental fits from two independent experiments shown in Fig. 5g.
