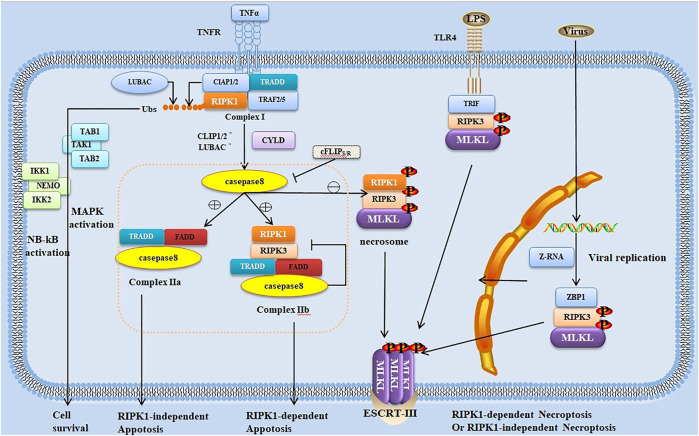
FIGURE 1.
Trigger and regulation of signal transduction pathways in necroptosis and apoptosis. Necroptosis typically occurs as a consequence of the stimulation of tumor necrosis factor receptor (TNFR) 1 by TNF, leading to the formation of receptor-bound complex I such as TNFR1-associated DD (TRADD), receptor-interacting protein (RIP) kinase (RIPK) 1, TNF-associated factor 2/5 (TRAF2/TRAF5), cellular inhibitor of apoptosis proteins (cIAPs), and linear ubiquitin chain assembly complex (LUBAC). RIPK1 ubiquitination by LUBAC or cIAPs results in the recruitment of I-κB kinase (IKK) complex (including IKK1, IKK2, and the nuclear factor-kappa B (NF-κB) essential modulator [NEMO]) and transforming growth factor-β-activated kinase 1 (TAK1)-binding protein (TAB) complex, leading to the activation of mitogen-activated protein kinase and NF-κB for cell survival. Cylindromatosis (CYLD) and A20 deubiquitinate RIPK1, which is released from complex I, leading to the transition from complex I to cytosolic complex II. The binding of TRADD–Fas-associated death domain protein (FADD)–caspase-8, one complex called complex IIa, triggers RIPK1-independent apoptosis, whereas the other complex IIb (comprising RIPK1, RIPK3, FADD, and caspase-8) triggers RIPK1-dependent apoptosis. Moreover, RIPK1 can inhibit FADD–caspase-8-dependent apoptosis. Caspase-8 can be inhibited by c-FLIP as a result of heterodimerization between c-FLIPS and procaspase-8. With the reduction, blockage, or absence of cIAPs or caspase-8, the necrosome (comprising RIPK1, RIPK3, and MLKL) is formed, which is the binding of activated RIPK1 and RIPK3, leading to the activation and phosphorylation of RIPK3. RIPK3 then activates MLKL, which is transferred to the cell membrane, ultimately leading to cell expansion and rupture and RIPK1-dependent necroptosis. RIPK3 can be activated not with RIPK1 but with other RIP homotypic interaction motif (RHIM)-containing proteins such as TLRs. TLRs can induce RIPK3 activation and RIPK1-independent necroptosis through the RHIM-containing adapter TIR domain-containing adaptor-inducing interferon-β (TRIF). In the nucleus of influenza virus-infected cells, Z-RNAs are produced when replicated viruses are sensed by the host Z-DNA-binding protein 1 (ZBP1), activating RIPK3 independent of RIPK1 by its RHIM domain.