Abstract
Prion protein has two isoforms including cellular prion protein (PrPC) and scrapie prion protein (PrPSc). PrPSc is the pathological aggregated form of prion protein and it plays an important role in neurodegenerative diseases. PrPC is a glycosylphosphatidylinositol (GPI)-anchored protein that can attach to a membrane. Its expression begins at embryogenesis and reaches the highest level in adulthood. PrPC is expressed in the neurons of the nervous system as well as other peripheral organs. Studies in recent years have disclosed the involvement of PrPC in various aspects of cancer biology. In this review, we provide an overview of the current understanding of the roles of PrPC in proliferation, cell survival, invasion/metastasis, and stem cells of cancer cells, as well as its role as a potential therapeutic target.
Keywords: cellular prion protein, cancer, proliferation, metastasis, drug resistance, cancer stem cell
Introduction
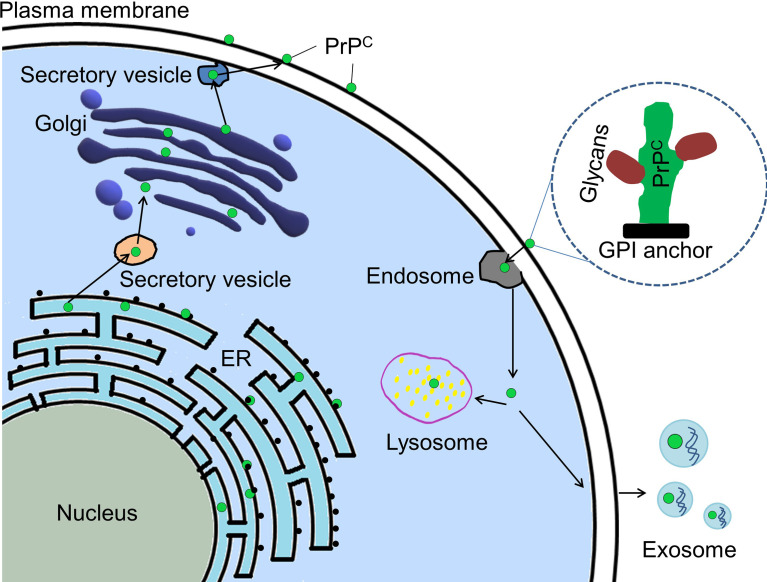
Prion protein (PrP) is expressed throughout the whole body. It has two isoforms, cellular prion protein (PrPC) and its pathogenic form-scrapie prion protein (PrPSc) (1, 2). PrPSc is well known for its ability to cause a series of neurodegenerative diseases in human and other mammals (1, 3). It results from post-translational conversion of the glycosylphosphatidylinositol (GPI)-anchored PrPC (4, 5). PrPC, as a scaffold on the cell surface, recruits different partners to execute its functions being involved in signaling pathways (6). The biosynthetic pathway of PrPC is similar to that of other membrane-attached and secreted proteins (5) (Figure 1). It is synthesized in endoplasmic reticulum (ER)-attached ribosomes followed by its import into ER where it is glycosylated and modified by GPI anchor before it is transported into Golgi for further modification. Then PrPC is transported to the cell surface where it can be internalized through endocytic pathway (7). The internalized PrPC can be transported into the lysosome for degradation or be enclosed in exosomes and secreted outside the cells (7). PrPC is mainly attached to lipid rafts on the cell surface via its C-terminal GPI anchor (8, 9). It is also located in the cytosol and the nucleus (10–12). Interestingly, PrPC was found in the exosomes secreted by cancer cells (13).
Figure 1.
Cellular trafficking pathway of PrPC. PrPC (green dot) is synthesized in ribosome attached to ER (endoplasmic reticulum). PrPC is imported to ER where it will be glycosylated and modified by GPI anchor before it is transported into Golgi apparatus for further modification. Mature PrPC is trafficked to plasma membrane and located there by its GPI anchor. Some mature PrPC could be endocytosed for degradation in the lysosome or for being contained in exosomes and secreted outside the cell. PrPC, Cellular prion protein; GPI, glycosylphosphatidylinositol.
Cancer is the second leading cause of death worldwide. Studies in recent years show that PrPC is involved in various aspects of cancer biology such as cell proliferation, metastasis, cell death, drug resistance and cancer stem cells (14–21). In this review, we summarize the current progress in these aspects.
PrPC Promotes Cancer Cell Proliferation
PrPC can promote proliferation in cancer cells (22). Liang et al. demonstrated that overexpression of PrPC promoted cell proliferation through activation of the phosphatidylinositide 3-kinase (PI3K) pathway and promotion of the G1/S phase transition by upregulating cyclin D1, in gastric cancer cells (22). PrPC is also involved in G1 to S phase transition in renal adenocarcinoma ACHN and colon adenocarcinoma LS 174T cells (23). Knockdown of PrPC inhibited cell proliferation and amplified the inhibitory effect of fucoidan on cell proliferation by suppressing expression of cyclins and cyclin-dependent kinase (CDK), in HT29 colon cancer cells (24). Interaction of PrPC with the co-chaperone Hsp70/90 organizing protein (HOP) promoted proliferation via activating PI3K and extracellular-signal-regulated kinase (ERK1/2) pathways in glioblastomas (GBM) cells (25). Furthermore, HOP-PrPC interaction promoted proliferation of glioblastoma stem-like cells and the decrease expression of PrPC and HOP may work as an effective therapy for GBM in the future (26). Warburg effect refers to the event that cancer cells preferentially use aerobic glycolysis to generate energy and reducing power for their biosynthesis, cell survival and proliferation (27). Overexpression of PrPC mediated Warburg effect by increasing glucose transporter 1 (Glut1) expression which promotes glucose uptake through epigenetic activation of Fyn-HIF-2α-Glut1 pathway in colorectal cancer cells (28). PrPC can also increase cell proliferation by interacting with 37/67 kDa non-integrin laminin receptor (LR/37/67 kDa) and activating downstream ERK1/2 and PI3K/protein kinase B (AKT) signaling pathways in schwannoma cells (29). It promoted proliferation by interacting with Notch1 in pancreatic ductal adenocarcinoma (PDAC) (30). A variant of PrPC with one octapeptide repeat deletion (1-OPRD) is widely present in gastric cancer cell lines and gastric cancer tissues (31). Overexpression of 1-OPRD could promote the proliferation of gastric cancer cells through transcriptional activation of cyclin D3, which facilitated the G1-/S-phase transition in cell cycle (32).
PrPC Promotes Cancer Cell Invasion/Metastasis
Metastasis leads to more than 90% of cancer-caused death, but its underlying mechanisms still remain poorly understood (33). Christine L et al. divided the process of metastasis into two phases: the first phase is physical translocation of cancer cell from a primary tumor to other distant tissues, and the second phase is colonization of metastatic cancer cells in their new microenvironment (33). EMT refers to epithelial-to-mesenchymal transition (34). Many in vitro models show that EMT act as a key process during cancer metastasis (35, 36). Transcription of Prnp (the gene encoding PrP) considerably increased during EMT (37). Upregulation of PrPC and dedifferentiation of EMT-like cells were observed in invasive colorectal cancer cells (CRC) (18, 38). Overexpression of PrPC by transfecting pCDNA3.0-Prnp in SW480 cells led to EMT whereas, knockdown of Prnp in mesenchymal-like LIM2405 cells caused MET (mesenchymal-to-epithelial transition) (18). The mechanisms underlying EMT enhancement by PrPC are largely unclear.
SATB1 (special AT-rich sequence-binding proteins 1) is a nuclear matrix associated protein. It can induce tumor metastasis by altering chromatin structure and upregulating metastasis-associated genes while downregulating tumour-suppressor genes (39, 40). Knockdown of Prnp resulted in loss of SATB1 expression and reduction of metastatic capacity in CRC with Fyn and specificity protein 1(SP1) being involved in this process, indicating that PrPC may promote tumor metastasis via upregulating the PrPC-Fyn-SP1-SATB1 axis (18). PrPC and γ-Syn are overexpressed in CRC (41, 42). They may be involved in colorectal cancer cell metastasis by inducing an endothelial proliferation to differentiation switch (42, 43).
PrPC is highly expressed in metastatic gastric cancer cells and it may promote invasion and metastasis through activation of the mitogen-activated protein kinases (MEK)/ERK pathway and consequent transactivation of matrix metalloproteinase-11(MMP11) (44). MMP11 can promote matrix degradation, inflammation and tissue remodeling (20, 44). Its N-terminal fragment is essential for transducing invasion-promoting signal of PrPC (20, 44). Tissue Inhibitor of Metalloproteinase (TIMP) is endogenous inhibitor for membrane type1-matrix metalloproteinase (MT1-MMP). The binding of TIMP to the GPI anchor of the prion protein generated a membrane-tethered, high-affinity designer TIMP (named “T1Pr αMT1” hereafter) which is expressed on the cell surface and co-localized with cellular MTI-MMP (45). Therefore, GPI anchor of PrPC might be used as a potential therapy for renal carcinoma (45).
It was reported that PrPC promoted EMT through the activation of the ERK2/mitogen-activated protein kinase (MAPK1) pathway in colorectal cancer stem cells (46). This is consistent with the notion that the appearance of the CSC (cancer stem cell) phenotype and EMT are intimately connected (19). Notch1 is involved in CSCs (47). It is a downstream effector of PrPC both of which colocalizes on the cell membrane and form an interaction network to promote pancreatic cancer cell metastasis (30). Co-treatment with 5-fluorouracil (5-FU) and melatonin could inhibit colon CSC marker octamer-binding transcription factor 4 (Oct4) via downregulation of PrPC-Oct4 pathways (48). Tumor-mediated angiogenesis will be suppressed in this process which suggests that cancer metastasis will be inhibited (48). PrPC-containing exosomes secreted by CRC could also promote tumor metastasis by increasing the permeability of endothelial cells and the secretion of angiogenic factors (49). This study also demonstrated that the combination of anti-PrPC and 5-FU downregulated tumor progression (49).
The immune system is one of the key pathways to control cancer development and metastasis. Regulatory T cells (Tregs), which have immunosuppressive activity (50), are one of the main targets of cancer immunotherapy (51). By constructing a lung metastatic model of melanoma in Prnp0/0 and Tga20 mice, it was demonstrated that the increased expression of PrPC induces the development of Tregs by upregulating transforming growth factor-beta (TGF-β) and programmed death ligand-1(PD-L1), thereby promoting tumor progression (52).
Many studies have demonstrated that PrPC expression promotes cancer cell metastasis (Figure 2). However, one study showed that knockout of Prnp (Prnp 0/0) in mesenchymal embryonic mouse cells transformed by Ras/Myc led to more incidence of lung metastasis due to increased expression of αVβ3-integrin (53). This suggest that more studies are required to clarify the roles of PrPC in cancer metastasis.
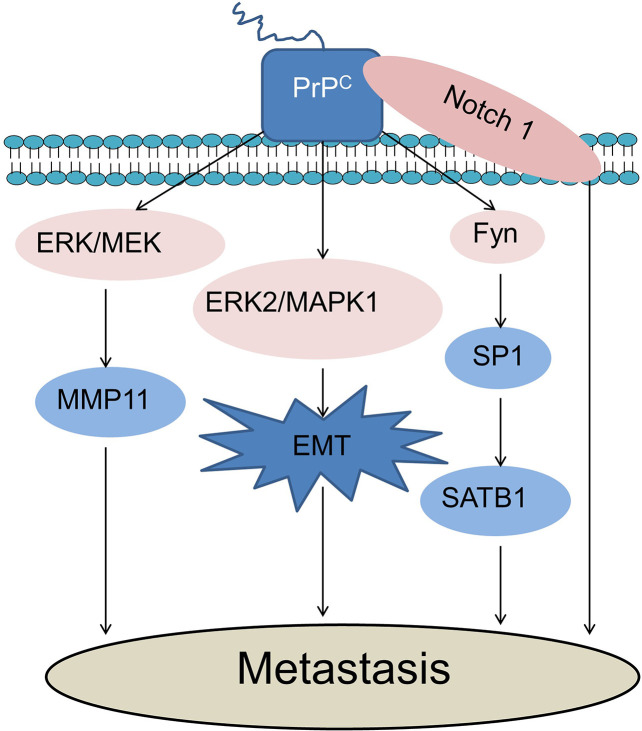
Figure 2.
PrPC promotes cancer cell metastasis. PrPC could promote cancer cell metastasis through activation of the MEK/ERK pathway and consequent transactivation of MMP11. PrPC promotes EMT through the activation of the ERK2/MAPK1 pathway during cancer metastasis. PrPC could promote tumor metastasis via up-regulating the PrPC-Fyn-SP1-SATB1 axis. Notch1 and PrPC could form an interaction network to promote cancer cell metastasis. ERK, Extracellular-signal-regulated kinase; MEK, Mitogen-activated protein kinases; MMP11, Matrix metalloproteinase-11; MAPK, Mitogen-activated protein kinase; EMT, Epithelial-mesenchymal transition; SATB1, Special AT-rich sequence-binding proteins 1; SP1, Specificity protein 1.
PrPC Promotes Cancer Cell Drug Resistance
One major challenge for cancer treatment is drug resistance. Various mechanisms can contribute to cancer drug resistance (54). The most studied mechanisms involving the roles of PrPC in cancer drug resistance include multi-drug resistance (MDR) and inhibition of cell death. Multi-drug resistance (MDR) refers to the ability of cancer cells to survive against a wide range of anti-cancer drugs (55). Cell death can be classified into three main types including apoptosis (Type I programmed cell death), autophagic cell death (Type II programmed cell death) and necrosis (56). Apoptosis is characterized by cell shrinkage, membrane blebbing, chromatin condensation, DNA fragmentation and caspase activation. Autophagic cell death is induced by the over-activation of autophagy that is an intracellular lysosomal degradation process. Necrosis is a non-programmed cell death. It is caused by sudden results to the cells and is characterized by breakage of plasma membrane followed by cytoplasmic leakage.
Upregulation of PrPC can lead to drug resistance in different types of cancers cells (57–59). In colorectal cancer cells, PrPC is involved in 5-FU resistance by increasing cell survival and proliferation via activating PI3K-Akt signaling pathway and the expression of cell cycle-associated proteins (59). PrPC overexpression led to resistance of colorectal cancer LS174T cells to doxorubicin-induced apoptosis by upregulation of the inhibitors of apoptosis proteins (IAPs) (60). Upregulation of PrPC leads to increased superoxide dismutase and catalase activities and decreased endoplasmic reticulum stress and apoptosis, which results in oxaliplatin resistance in colorectal cancer cells (61, 62). In gastric cancer cells, PrPC can promote drug resistance by different mechanisms. PrPC coexists with MGr1-Antigen/37 kDa laminin receptor precursor (MGr1-Ag/37LRP) to promote MDR in gastric cancer cells by inhibiting apoptosis via activation of the PI3K/AKT signaling pathway (63). Octarepeat peptides of PrP may be involved in gastric cancer MDR by increasing the activities of antioxidant enzymes (64). PrPC can promote MDR by upregulating the multidrug resistance protein (P-gp) and suppressing apoptosis in gastric and breast cancer cells (65, 66). Overexpression of PrPC promotes resistance to TNF-α-induced apoptosis by inhibiting Bcl-2-associated X protein (Bax) expression in renal adenocarcinoma ACHN cells (23).
PrPC can be found on the cell surface by attaching to the cell membrane and outside the cells being contained in exosomes which are secreted from the cells (67, 68). The secreted PrPC in tumor microenvironment binds to doxorubicin to prevent it from entering the nucleus and intercalating into DNA to induce cell death; and breast cancer patients with high levels of serum PrPC are at high risk of relapse following doxorubicin treatment (13). PrP synthetic peptide (amino acid residues 105 - 120 of the human prion protein) can protect schwannoma cells from H2O2-mediated cell death (29).
PrPC has been shown to protect cancer cells from apoptosis and autophagic cell death (69). PrPC inhibits apoptosis in neurons and in cancer cells (70). PrPC upregulation inhibits apoptosis induced by Bax expression, serum starvation and anti-cancer drug treatments (57, 70, 71). PrPC can bind to the C-terminus of the anti-apoptotic protein Bcl-2 to form a dimer inhibiting apoptosis (72). When PrPC is upregulated, Bcl-2/Bax ratio increases, resulting in anti-apoptosis in breast carcinoma MCF-7 cells (71). Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a ligand for death receptors which can induce cancer cell apoptosis (73). Downregulation of PrPC sensitizes adriamycin-resistant human breast cancer cells to TRAIL-induced apoptosis by increasing Bax/Bcl-2 ratio (58). PrPC inhibited TRAIL-induced apoptosis under hypoxia in human colon carcinoma cells (74). Akt was activated by PrPC to prevent TRAIL-induced apoptosis (75, 76). PrPC also activated PI3K/Akt signaling pathway contributing to its anti-Bax function by preventing the pro-apoptotic conformational changes of Bax at the early step of Bax activation (71). Moreover, PrPC protected lung and pancreatic cancer cells from apoptosis through downregulation of unfolded protein response (UPR) (77).
Autophagy is an evolutionarily conserved catabolic process in eukaryotic cells, in which unnecessary or dysfunctional cytosolic components are degraded and recycled through lysosomes (78). During autophagy (macroautophagy), cytosolic components (cargos) are surrounded by a phagophore which will expands and encloses to form the characteristic double-membraned structure autophagosome. Then, autophagosome will fuse with the lysosome to form autolysosome where cargos are degraded to generate small molecules that can be used for biosynthesis and energy production for cell survival, under stress conditions such as starvation (79). However, when autophagy is over-enhanced, it can induce cell death (autophagic cell death/autophagy-induced cell death) (79). Barbieri et al. demonstrated for the first time that PrPC can modulate autophagic cell death in glial tumor cells (80). They demonstrated that PrPC silencing resulted in inhibition of Mammalian target of rapamycin (mTOR) kinase activity in T98G glioma cells, promoting autophagy leading to autophagic cell death (80). Furthermore, PrPC inhibited autophagy by activating the antioxidant enzyme SOD (81). Since autophagy is mainly a pro-cell survival mechanism, it is expected that PrPC may antagonize drug resistance by inhibiting autophagy in cancer cells.
One study showed that tumor resistance to radiotherapy was also associated with the increased PrPC (82). In neuroblastoma, breast, and colorectal cancer cell lines, ionizing radiation (IR) can increase the expression of PrPC by activating ATM-TAK1-PrPC pathway, thereby leading to the resistance to radiotherapy of tumor cells (82). Taken together, PrPC can modulate various signaling pathways contributing to cancer drug resistance (Figure 3).
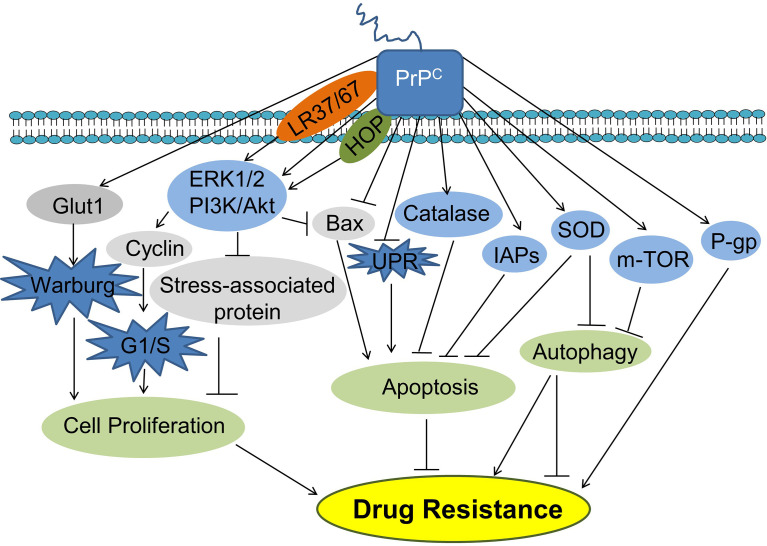
Figure 3.
PrPC promotes cancer cell drug resistance. PrPC can promote cancer cell drug resistance by promoting cell proliferation and inhibiting apoptosis. PrPC can also suppress autophagy inhibiting or promoting drug resistance. HOP, Hsp70/90 organizing protein; IAPs, Inhibitors of apoptosis proteins; Glut1, Glucose transporter 1; PI3K, Phosphatidylinositide 3-kinase; AKT, Protein kinase B; Bax, Bcl-2-associated X protein; UPR, Unfolded protein response; SOD, Superoxide dismutase; P-gp, P-glycoprotein.
Although the overexpression of PrPC in cancer cells results in therapy-resistance, researchers have taken advantage of this characteristic to synthesize PrPC-Apt-functionalized doxorubicin-oligomer-AuNPs (PrPC-AptDOa) which could target PrPC-overexpressed CRC (83). PrPC-AptDOa inhibited CRCs proliferation and induced apoptosis more significantly than free Dox at the cellular level (83). However, PrPC is also expressed in normal cells, such as neurons and neuroglia. Therefore, the challenge for cancer treatment is to specifically target PrPC in cancer cells. In addition, further studies of PrPC-AptDOa should be conducted in an animal model and clinical trials to clarify its therapeutic effects and side effects on individuals.
PrPC Promotes Cancer Stem Cell Development
Cancer stem cells (CSCs) are a small subpopulation of cancer cells with the capacities of self-renewal, differentiation and tumorigenicity (84). PrPC is engaged in different types of stem cells, such as hematopoietic stem cells (HSCs), gland stem cells, bone marrow-derived human mesenchymal stem cells (MSCs) and human embryonic stem(ES) cells (85–88). Studies have indicated that PrPC is also involved in CSCs. PrPC protected Oct4, a marker of colon cancer stem cells, from degradation by inducing heat shock protein 1 like (HSPA1L) when in response to co‐treatment with 5‐FU and melatonin (48). One study indicated that PrPC was highly expressed in consensus molecular subgroup (CMS4), a subtype of CRC with higher malignancy, and affected the prognosis of CRC as an upstream molecule in the PrPC-ILK-IDO1 axis (89). PrPC promoted EMT of colorectal cancer stem cells via activation of the ERK2 (MAPK1) pathway to increase cell metastasis (46). CD44 is a CSC marker and critical regulator of cancer stemness (90). PrPC is co-expressed with CD44 in colorectal CSCs (46). PrPC and Hsp70/90 organizing protein (HOP) acted together to regulate self-renewal, proliferation and migration in glioblastoma (GBM) stem-like cells (26). Downregulation of PrPC decreased stem cell-like properties of human GBM CSCs (91). Downregulation of PrPC in models of prion disease through immune, genetic and other mechanisms has achieved some progress. Application of anti-PrP antibodies have been proposed as a promising treatment many decades ago (92, 93). A recent study reported that transgenic mice expressing elk PrP (TgElk) benefited from active PrP vaccination (94). Minikel et al. demonstrated that PrP-lowering antisense oligonucleotides (ASOs) worked via an RNAase-H dependent mechanism and has certain therapeutic effect on prion-infected mice (95). Minikel et al. also proposed that loss-of-function variant of Prnp could be potential targets for prion disease inhibitory drugs (96). The application of these PrPC-lowering approaches may provide novel cancer therapies by targeting CSCs.
Conclusion
Prion protein (PrP) is expressed in nervous system and other organs (97). There are two forms of PrP, including normal PrPC and disease causing PrPSc. PrPC misfolding and aggregation can cause fatal neurodegenerative conditions (98). Studies in recent years show that it also plays a role in cancer. PrPC can stimulate cancer progression by promoting cancer cell proliferation, invasion/metastasis, drug resistance, and cancer stem cell development. Therefore, targeting PrPC is a novel approach for cancer treatment.
Author Contributions
MD and YC conceived the topic and designed the outline of this review. MD contributed to the manuscript writing and prepared the figures and tables. YC modified the language. LC, YC and YL critically revised the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (82071351).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
Bax, Bcl-2-associated X protein; Bcl, B cell leukemia oncogene; CRC, Colorectal cancer cell; CSC, Cancer stem cell; EMT, Epithelial-Mesenchymal Transition; ERK, Extracellular-signal-regulated kinase; 5-FU, 5-fluorouracil; GBM, Glioblastomas; GPI, Glycosylphosphatidylinositol; HOP, Hsp70/90 organizing protein; HSCs, Hematopoietic stem cells; MDR, Multi-drug resistance; MET, Mesenchymal-to-epithelial transition; MMP11, Matrix metalloproteinase-11; MSCs, Mesenchymal stem cells; Oct4, Octamer-binding transcription factor 4; 1-OPRD, One octapeptide repeat deletion; PDAC, Pancreatic ductal adenocarcinoma; P-gp, P-glycoprotein; PI3K, Phosphatidylinositol 3 kinase; PrPC, Cellular prion protein; PrPSc, Scrapie prion protein; PrPC-AptDOa, PrPC-Apt-functionalized doxorubicin-oligomer-AuNPs; SATB1, Special AT-rich sequence-binding proteins 1; SOD, Superoxide dismutase; TIMP, Tissue Inhibitor of Metalloproteinase; TNF-α, Tumor Necrosis Factor-α; TRAIL, Tumor necrosis factor-related apoptosis-inducing ligand; UPR, Unfolded protein response.
References
- 1.Atkinson CJ, Zhang K, Munn AL, Wiegmans A, Wei MQ. Prion Protein Scrapie and the Normal Cellular Prion Protein. Prion (2016) 10(1):63–82. doi: 10.1080/19336896.2015.1110293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priola SA, Chesebro B, Caughey B. Biomedicine. A View From the Top–Prion Diseases From 10,000 Feet. Sci (N Y NY) (2003) 300(5621):917–9. doi: 10.1126/science.1085920 [DOI] [PubMed] [Google Scholar]
- 3.Soto C, Satani N. The Intricate Mechanisms of Neurodegeneration in Prion Diseases. Trends Mol Med (2011) 17(1):14–24. doi: 10.1016/j.molmed.2010.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westergard L, Christensen HM, Harris DA. The Cellular Prion Protein (PrP(C)): Its Physiological Function and Role in Disease. Biochim Biophys Acta (2007) 1772(6):629–44. doi: 10.1016/j.bbadis.2007.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harris DA. Trafficking, Turnover and Membrane Topology of PrP. Br Med Bull (2003) 66:71–85. doi: 10.1093/bmb/66.1.71 [DOI] [PubMed] [Google Scholar]
- 6.Linden R, Cordeiro Y, Lima LMTR. Allosteric Function and Dysfunction of the Prion Protein. Cell Mol Life Sci CMLS (2012) 69(7):1105–24. doi: 10.1007/s00018-011-0847-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alves RN, Iglesia RP, Prado MB, Melo Escobar MI, Boccacino JM, Fernandes CFL, et al. A New Take on Prion Protein Dynamics in Cellular Trafficking. Int J Mol Sci (2020) 21(20):7763. doi: 10.3390/ijms21207763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stahl N, Borchelt DR, Hsiao K, Prusiner SB. Scrapie Prion Protein Contains a Phosphatidylinositol Glycolipid. Cell (1987) 51(2):229–40. doi: 10.1016/0092-8674(87)90150-4 [DOI] [PubMed] [Google Scholar]
- 9.Naslavsky N, Stein R, Yanai A, Friedlander G, Taraboulos A. Characterization of Detergent-Insoluble Complexes Containing the Cellular Prion Protein and its Scrapie Isoform. J Biol Chem (1997) 272(10):6324–31. doi: 10.1074/jbc.272.10.6324 [DOI] [PubMed] [Google Scholar]
- 10.Fioriti L, Dossena S, Stewart LR, Stewart RS, Harris DA, Forloni G, et al. Cytosolic Prion Protein (PrP) Is Not Toxic in N2a Cells and Primary Neurons Expressing Pathogenic PrP Mutations. J Biol Chem (2005) 280(12):11320–8. doi: 10.1074/jbc.M412441200 [DOI] [PubMed] [Google Scholar]
- 11.Déry M-A, Jodoin J, Ursini-Siegel J, Aleynikova O, Ferrario C, Hassan S, et al. Endoplasmic Reticulum Stress Induces PRNP Prion Protein Gene Expression in Breast Cancer. Breast Cancer Res BCR (2013) 15(2):R22–R. doi: 10.1186/bcr3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morel E, Fouquet S, Strup-Perrot C, Pichol Thievend C, Petit C, Loew D, et al. The Cellular Prion Protein PrP(c) Is Involved in the Proliferation of Epithelial Cells and in the Distribution of Junction-Associated Proteins. PloS One (2008) 3(8):e3000–e. doi: 10.1371/journal.pone.0003000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wiegmans AP, Saunus JM, Ham S, Lobb R, Kutasovic JR, Dalley AJ, et al. Secreted Cellular Prion Protein Binds Doxorubicin and Correlates With Anthracycline Resistance in Breast Cancer. JCI Insight (2019) 5(6):e124092. doi: 10.1172/jci.insight.124092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santos TG, Lopes MH, Martins VR. Targeting Prion Protein Interactions in Cancer. Prion (2015) 9(3):165–73. doi: 10.1080/19336896.2015.1027855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehrpour M, Codogno P. Prion Protein: From Physiology to Cancer Biology. Cancer Lett (2010) 290(1):1–23. doi: 10.1016/j.canlet.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 16.Tang Z, Ma J, Zhang W, Gong C, He J, Wang Y, et al. The Role of Prion Protein Expression in Predicting Gastric Cancer Prognosis. J Cancer (2016) 7(8):984–90. doi: 10.7150/jca.14237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim J-I, Cali I, Surewicz K, Kong Q, Raymond GJ, Atarashi R, et al. Mammalian Prions Generated From Bacterially Expressed Prion Protein in the Absence of Any Mammalian Cofactors. J Biol Chem (2010) 285(19):14083–7. doi: 10.1074/jbc.C110.113464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Q, Qian J, Wang F, Ma Z. Cellular Prion Protein Accelerates Colorectal Cancer Metastasis via the Fyn-SP1-SATB1 Axis. Oncol Rep (2012) 28(6):2029–34. doi: 10.3892/or.2012.2025 [DOI] [PubMed] [Google Scholar]
- 19.Singh A, Settleman J. EMT, Cancer Stem Cells and Drug Resistance: An Emerging Axis of Evil in the War on Cancer. Oncogene (2010) 29(34):4741–51. doi: 10.1038/onc.2010.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duffy MJ, Maguire TM, Hill A, McDermott E, O’Higgins N. Metalloproteinases: Role in Breast Carcinogenesis, Invasion and Metastasis. Breast Cancer Res BCR (2000) 2(4):252–7. doi: 10.1186/bcr65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Medema JP. Cancer Stem Cells: The Challenges Ahead. Nat Cell Biol (2013) 15(4):338–44. doi: 10.1038/ncb2717 [DOI] [PubMed] [Google Scholar]
- 22.Liang J, Pan Y, Zhang D, Guo C, Shi Y, Wang J, et al. Cellular Prion Protein Promotes Proliferation and G1/S Transition of Human Gastric Cancer Cells SGC7901 and AGS. FASEB J Off Publ Fed Am Soc Exp Biol (2007) 21(9):2247–56. doi: 10.1096/fj.06-7799com [DOI] [PubMed] [Google Scholar]
- 23.Yap YH-Y, Say Y-H. Resistance Against Tumour Necrosis Factor α Apoptosis by the Cellular Prion Protein Is Cell-Specific for Oral, Colon and Kidney Cancer Cell Lines. Cell Biol Int (2012) 36(3):273–7. doi: 10.1042/CBI20110088 [DOI] [PubMed] [Google Scholar]
- 24.Yun CW, Yun S, Lee JH, Han Y-S, Yoon YM, An D, et al. Silencing Prion Protein in HT29 Human Colorectal Cancer Cells Enhances Anticancer Response to Fucoidan. Anticancer Res (2016) 36(9):4449–58. doi: 10.21873/anticanres.10989 [DOI] [PubMed] [Google Scholar]
- 25.Lopes MH, Santos TG, Rodrigues BR, Queiroz-Hazarbassanov N, Cunha IW, Wasilewska-Sampaio AP, et al. Disruption of Prion Protein-HOP Engagement Impairs Glioblastoma Growth and Cognitive Decline and Improves Overall Survival. Oncogene (2015) 34(25):3305–14. doi: 10.1038/onc.2014.261 [DOI] [PubMed] [Google Scholar]
- 26.Iglesia RP, Prado MB, Cruz L, Martins VR, Santos TG, Lopes MH. Engagement of Cellular Prion Protein With the Co-Chaperone Hsp70/90 Organizing Protein Regulates the Proliferation of Glioblastoma Stem-Like Cells. Stem Cell Res Ther (2017) 8(1):76–. doi: 10.1186/s13287-017-0518-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Warburg O. The Metabolism of Carcinoma Cells. J Cancer Res (1925) 9:148–63. doi: 10.1158/jcr.1925.148 [DOI] [Google Scholar]
- 28.Li Q-Q, Sun Y-P, Ruan C-P, Xu X-Y, Ge J-H, He J, et al. Cellular Prion Protein Promotes Glucose Uptake Through the Fyn-HIF-2α-Glut1 Pathway to Support Colorectal Cancer Cell Survival. Cancer Sci (2011) 102(2):400–6. doi: 10.1111/j.1349-7006.2010.01811.x [DOI] [PubMed] [Google Scholar]
- 29.Provenzano L, Ryan Y, Hilton DA, Lyons-Rimmer J, Dave F, Maze EA, et al. Cellular Prion Protein (PrPc) in the Development of Merlin-Deficient Tumours. Oncogene (2017) 36(44):6132–42. doi: 10.1038/onc.2017.200 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y, Yu S, Huang D, Cui M, Hu H, Zhang L, et al. Cellular Prion Protein Mediates Pancreatic Cancer Cell Survival and Invasion Through Association With and Enhanced Signaling of Notch1. Am J Pathol (2016) 186(11):2945–56. doi: 10.1016/j.ajpath.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang J, Wang JB, Pan YL, Wang J, Liu LL, Guo XY, et al. High Frequency Occurrence of 1-OPRD Variant of PRNP Gene in Gastric Cancer Cell Lines and Chinese Population With Gastric Cancer. Cell Biol Int (2006) 30(11):920–3. doi: 10.1016/j.cellbi.2006.05.015 [DOI] [PubMed] [Google Scholar]
- 32.Liang J, Wang J, Luo G, Pan Y, Wang X, Guo C, et al. Function of PrPC (1-OPRD) in Biological Activities of Gastric Cancer Cell Lines. J Cell Mol Med (2009) 13(11-12):4453–64. doi: 10.1111/j.1582-4934.2009.00687.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chaffer CL, Weinberg RA. A Perspective on Cancer Cell Metastasis. Sci (N Y NY) (2011) 331(6024):1559–64. doi: 10.1126/science.1203543 [DOI] [PubMed] [Google Scholar]
- 34.Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-Mesenchymal Transitions in Development and Disease. Cell (2009) 139(5):871–90. doi: 10.1016/j.cell.2009.11.007 [DOI] [PubMed] [Google Scholar]
- 35.Mittal V. Epithelial Mesenchymal Transition in Tumor Metastasis. Annu Rev Pathol (2018) 13:395–412. doi: 10.1146/annurev-pathol-020117-043854 [DOI] [PubMed] [Google Scholar]
- 36.Nieto MA. The Ins and Outs of the Epithelial to Mesenchymal Transition in Health and Disease. Annu Rev Cell Dev Biol (2011) 27:347–76. doi: 10.1146/annurev-cellbio-092910-154036 [DOI] [PubMed] [Google Scholar]
- 37.Mehrabian M, Brethour D, Wang H, Xi Z, Rogaeva E, Schmitt-Ulms G. The Prion Protein Controls Polysialylation of Neural Cell Adhesion Molecule 1 During Cellular Morphogenesis. PloS One (2015) 10(8):e0133741–e. doi: 10.1371/journal.pone.0133741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Natalwala A, Spychal R, Tselepis C. Epithelial-Mesenchymal Transition Mediated Tumourigenesis in the Gastrointestinal Tract. World J Gastroenterol (2008) 14(24):3792–7. doi: 10.3748/wjg.14.3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Han H-J, Russo J, Kohwi Y, Kohwi-Shigematsu T. SATB1 Reprogrammes Gene Expression to Promote Breast Tumour Growth and Metastasis. Nature (2008) 452(7184):187–93. doi: 10.1038/nature06781 [DOI] [PubMed] [Google Scholar]
- 40.Pavan Kumar P, Purbey PK, Sinha CK, Notani D, Limaye A, Jayani RS, et al. Phosphorylation of SATB1, a Global Gene Regulator, Acts as a Molecular Switch Regulating Its Transcriptional Activity In Vivo . Mol Cell (2006) 22(2):231–43. doi: 10.1016/j.molcel.2006.03.010 [DOI] [PubMed] [Google Scholar]
- 41.Antonacopoulou AG, Grivas PD, Skarlas L, Kalofonos M, Scopa CD, Kalofonos HP. POLR2F, ATP6V0A1 and PRNP Expression in Colorectal Cancer: New Molecules With Prognostic Significance? Anticancer Res (2008) 28(2B):1221–7. [PubMed] [Google Scholar]
- 42.Ong S-H, Goh K-W, Chieng CK-L, Say Y-H. Cellular Prion Protein and γ-Synuclein Overexpression in LS 174T Colorectal Cancer Cell Drives Endothelial Proliferation-to-Differentiation Switch. PeerJ (2018) 6:e4506–e. doi: 10.7717/peerj.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bussolino F, Mantovani A, Persico G. Molecular Mechanisms of Blood Vessel Formation. Trends Biochem Sci (1997) 22(7):251–6. doi: 10.1016/s0968-0004(97)01074-8 [DOI] [PubMed] [Google Scholar]
- 44.Pan Y, Zhao L, Liang J, Liu J, Shi Y, Liu N, et al. Cellular Prion Protein Promotes Invasion and Metastasis of Gastric Cancer. FASEB J Off Publ Fed Am Soc Exp Biol (2006) 20(11):1886–8. doi: 10.1096/fj.06-6138fje [DOI] [PubMed] [Google Scholar]
- 45.Jiang B, Liu J, Lee MH. Targeting a Designer TIMP-1 to the Cell Surface for Effective MT1-MMP Inhibition: A Potential Role for the Prion Protein in Renal Carcinoma Therapy. Mol (Basel Switzerland) (2019) 24(2):255. doi: 10.3390/molecules24020255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Du L, Rao G, Wang H, Li B, Tian W, Cui J, et al. CD44-Positive Cancer Stem Cells Expressing Cellular Prion Protein Contribute to Metastatic Capacity in Colorectal Cancer. Cancer Res (2013) 73(8):2682–94. doi: 10.1158/0008-5472.CAN-12-3759 [DOI] [PubMed] [Google Scholar]
- 47.Xiao W, Gao Z, Duan Y, Yuan W, Ke Y. Notch Signaling Plays a Crucial Role in Cancer Stem-Like Cells Maintaining Stemness and Mediating Chemotaxis in Renal Cell Carcinoma. J Exp Clin Cancer Res (2017) 36(1):41–. doi: 10.1186/s13046-017-0507-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee JH, Yun CW, Han Y-S, Kim S, Jeong D, Kwon HY, et al. Melatonin and 5-Fluorouracil Co-Suppress Colon Cancer Stem Cells by Regulating Cellular Prion Protein-Oct4 Axis. J Pineal Res (2018) 65(4):e12519–e. doi: 10.1111/jpi.12519 [DOI] [PubMed] [Google Scholar]
- 49.Yun CW, Lee JH, Go G, Jeon J, Yoon S, Lee SH. Prion Protein of Extracellular Vesicle Regulates the Progression of Colorectal Cancer. Cancers (Basel) (2021) 13(9):2144. doi: 10.3390/cancers13092144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nishikawa H, Sakaguchi S. Regulatory T Cells in Cancer Immunotherapy. Curr Opin Immunol (2014) 27:1–7. doi: 10.1016/j.coi.2013.12.005 [DOI] [PubMed] [Google Scholar]
- 51.Colombo MP, Piconese S. Regulatory-T-Cell Inhibition Versus Depletion: The Right Choice in Cancer Immunotherapy. Nat Rev Cancer (2007) 7(11):880–7. doi: 10.1038/nrc2250 [DOI] [PubMed] [Google Scholar]
- 52.Cha S, Sin M-J, Kim M-J, Kim H-J, Kim Y-S, Choi E-K, et al. Involvement of Cellular Prion Protein in Invasion and Metastasis of Lung Cancer by Inducing Treg Cell Development. Biomolecules (2021) 11(2):285. doi: 10.3390/biom11020285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muras AG, Hajj GNM, Ribeiro KB, Nomizo R, Nonogaki S, Chammas R, et al. Prion Protein Ablation Increases Cellular Aggregation and Embolization Contributing to Mechanisms of Metastasis. Int J Cancer (2009) 125(7):1523–31. doi: 10.1002/ijc.24425 [DOI] [PubMed] [Google Scholar]
- 54.Mansoori B, Mohammadi A, Davudian S, Shirjang S, Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Advanced Pharm Bull (2017) 7(3):339–48. doi: 10.15171/apb.2017.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zahreddine H, Borden KLB. Mechanisms and Insights Into Drug Resistance in Cancer. Front Pharmacol (2013) 4:28. doi: 10.3389/fphar.2013.00028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Green DR, Llambi F. Cell Death Signaling. Cold Spring Harbor Perspect Biol (2015) 7(12):a006080. doi: 10.1101/cshperspect.a006080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang J-H, Du J-P, Zhang Y-H, Zhao X-J, Fan R-Y, Wang Z-H, et al. Dynamic Changes and Surveillance Function of Prion Protein Expression in Gastric Cancer Drug Resistance. World J Gastroenterol (2011) 17(35):3986–93. doi: 10.3748/wjg.v17.i35.3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Meslin F, Hamaï A, Gao P, Jalil A, Cahuzac N, Chouaib S, et al. Silencing of Prion Protein Sensitizes Breast Adriamycin-Resistant Carcinoma Cells to TRAIL-Mediated Cell Death. Cancer Res (2007) 67(22):10910–9. doi: 10.1158/0008-5472.CAN-07-0512 [DOI] [PubMed] [Google Scholar]
- 59.Lee JH, Yun CW, Lee SH. Cellular Prion Protein Enhances Drug Resistance of Colorectal Cancer Cells via Regulation of a Survival Signal Pathway. Biomol Ther (2018) 26(3):313–21. doi: 10.4062/biomolther.2017.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chieng CK-L, Say Y-H. Cellular Prion Protein Contributes to LS 174T Colon Cancer Cell Carcinogenesis by Increasing Invasiveness and Resistance Against Doxorubicin-Induced Apoptosis. Tumour Biol J Int Soc Oncodevelopmental Biol Med (2015) 36(10):8107–20. doi: 10.1007/s13277-015-3530-z [DOI] [PubMed] [Google Scholar]
- 61.Lee JH, Yoon YM, Han Y-S, Yun CW, Lee SH. Melatonin Promotes Apoptosis of Oxaliplatin-Resistant Colorectal Cancer Cells Through Inhibition of Cellular Prion Protein. Anticancer Res (2018) 38(4):1993–2000. doi: 10.21873/anticanres.12437 [DOI] [PubMed] [Google Scholar]
- 62.Brown DR, Schulz-Schaeffer WJ, Schmidt B, Kretzschmar HA. Prion Protein-Deficient Cells Show Altered Response to Oxidative Stress Due to Decreased SOD-1 Activity. Exp Neurol (1997) 146(1):104–12. doi: 10.1006/exnr.1997.6505 [DOI] [PubMed] [Google Scholar]
- 63.Luo G, Wang W, Wu Q, Lu Y, Su T, Gu N, et al. MGr1-Antigen/37 kDa Laminin Receptor Precursor Promotes Cellular Prion Protein Induced Multi-Drug-Resistance of Gastric Cancer. Oncotarget (2017) 8(42):71630–41. doi: 10.18632/oncotarget.17795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang JH, Du JP, Li SJ, Zhai LP, Yang XY, Wang ZH, et al. Octarepeat Peptides of Prion are Essential for Multidrug Resistance in Gastric Cancer Cells. J Digestive Dis (2012) 13(3):143–52. doi: 10.1111/j.1751-2980.2011.00563.x [DOI] [PubMed] [Google Scholar]
- 65.Li QQ, Cao XX, Xu JD, Chen Q, Wang WJ, Tang F, et al. The Role of P-Glycoprotein/Cellular Prion Protein Interaction in Multidrug-Resistant Breast Cancer Cells Treated With Paclitaxel. Cell Mol Life Sci CMLS (2009) 66(3):504–15. doi: 10.1007/s00018-008-8548-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Du J, Pan Y, Shi Y, Guo C, Jin X, Sun L, et al. Overexpression and Significance of Prion Protein in Gastric Cancer and Multidrug-Resistant Gastric Carcinoma Cell Line SGC7901/ADR. Int J Cancer (2005) 113(2):213–20. doi: 10.1002/ijc.20570 [DOI] [PubMed] [Google Scholar]
- 67.Hay B, Prusiner SB, Lingappa VR. Evidence for a Secretory Form of the Cellular Prion Protein. Biochemistry (1987) 26(25):8110–5. doi: 10.1021/bi00399a014 [DOI] [PubMed] [Google Scholar]
- 68.Lewis V, Johanssen VA, Crouch PJ, Klug GM, Hooper NM, Collins SJ. Prion Protein "Gamma-Cleavage": Characterizing a Novel Endoproteolytic Processing Event. Cell Mol Life Sci CMLS (2016) 73(3):667–83. doi: 10.1007/s00018-015-2022-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X, Zhang Y, Zhang L, He T, Zhang J, Li C. Prion Protein and Cancers. Acta Biochim Biophys Sin (Shanghai) (2014) 46(6):431–40. doi: 10.1093/abbs/gmu019 [DOI] [PubMed] [Google Scholar]
- 70.Kuwahara C, Takeuchi AM, Nishimura T, Haraguchi K, Kubosaki A, Matsumoto Y, et al. Prions Prevent Neuronal Cell-Line Death. Nature (1999) 400(6741):225–6. doi: 10.1038/22241 [DOI] [PubMed] [Google Scholar]
- 71.Roucou X, Giannopoulos PN, Zhang Y, Jodoin J, Goodyer CG, LeBlanc A. Cellular Prion Protein Inhibits Proapoptotic Bax Conformational Change in Human Neurons and in Breast Carcinoma MCF-7 Cells. Cell Death Differentiation (2005) 12(7):783–95. doi: 10.1038/sj.cdd.4401629 [DOI] [PubMed] [Google Scholar]
- 72.Kurschner C, Morgan JI. Analysis of Interaction Sites in Homo- and Heteromeric Complexes Containing Bcl-2 Family Members and the Cellular Prion Protein. Brain Res Mol Brain Res (1996) 37(1-2):249–58. doi: 10.1016/0169-328x(95)00323-k [DOI] [PubMed] [Google Scholar]
- 73.Wiley SR, Schooley K, Smolak PJ, Din WS, Huang CP, Nicholl JK, et al. Identification and Characterization of a New Member of the TNF Family That Induces Apoptosis. Immunity (1995) 3(6):673–82. doi: 10.1016/1074-7613(95)90057-8 [DOI] [PubMed] [Google Scholar]
- 74.Park J-Y, Jeong J-K, Lee J-H, Moon J-H, Kim S-W, Lee Y-J, et al. Induction of Cellular Prion Protein (PrPc) Under Hypoxia Inhibits Apoptosis Caused by TRAIL Treatment. Oncotarget (2015) 6(7):5342–53. doi: 10.18632/oncotarget.3028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Xu J, Zhou J-Y, Wei W-Z, Wu GS. Activation of the Akt Survival Pathway Contributes to TRAIL Resistance in Cancer Cells. PloS One (2010) 5(4):e10226–e. doi: 10.1371/journal.pone.0010226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ramljak S, Herlyn H, Zerr I. Cellular Prion Protein (PrPc) and Hypoxia: True to Each Other in Good Times and in Bad, in Sickness, and in Health. Front Cell Neurosci (2016) 10:292. doi: 10.3389/fncel.2016.00292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Gao Z, Peng M, Chen L, Yang X, Li H, Shi R, et al. Prion Protein Protects Cancer Cells Against Endoplasmic Reticulum Stress Induced Apoptosis. Virol Sin (2019) 34(2):222–34. doi: 10.1007/s12250-019-00107-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Alirezaei M, Kemball CC, Flynn CT, Wood MR, Whitton JL, Kiosses WB. Short-Term Fasting Induces Profound Neuronal Autophagy. Autophagy (2010) 6(6):702–10. doi: 10.4161/auto.6.6.12376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen Y, Klionsky DJ. The Regulation of Autophagy - Unanswered Questions. J Cell Sci (2011) 124(Pt 2):161–70. doi: 10.1242/jcs.064576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barbieri G, Palumbo S, Gabrusiewicz K, Azzalin A, Marchesi N, Spedito A, et al. Silencing of Cellular Prion Protein (PrPC) Expression by DNA-Antisense Oligonucleotides Induces Autophagy-Dependent Cell Death in Glioma Cells. Autophagy (2011) 7(8):840–53. doi: 10.4161/auto.7.8.15615 [DOI] [PubMed] [Google Scholar]
- 81.Oh J-M, Choi E-K, Carp RI, Kim Y-S. Oxidative Stress Impairs Autophagic Flux in Prion Protein-Deficient Hippocampal Cells. Autophagy (2012) 8(10):1448–61. doi: 10.4161/auto.21164 [DOI] [PubMed] [Google Scholar]
- 82.Bernardino-Sgherri J, Siberchicot C, Auvré F, Busso D, Brocas C, El Masri G, et al. Tumor Resistance to Radiotherapy Is Triggered by an ATM/TAK1-Dependent-Increased Expression of the Cellular Prion Protein. Oncogene (2021) 40(19):3460–9. doi: 10.1038/s41388-021-01746-0 [DOI] [PubMed] [Google Scholar]
- 83.Go G, Lee SH. The Cellular Prion Protein: A Promising Therapeutic Target for Cancer. Int J Mol Sci (2020) 21(23):9208. doi: 10.3390/ijms21239208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yu Z, Pestell TG, Lisanti MP, Pestell RG. Cancer Stem Cells. Int J Biochem Cell Biol (2012) 44(12):2144–51. doi: 10.1016/j.biocel.2012.08.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang CC, Steele AD, Lindquist S, Lodish HF. Prion Protein Is Expressed on Long-Term Repopulating Hematopoietic Stem Cells and Is Important for Their Self-Renewal. Proc Natl Acad Sci USA (2006) 103(7):2184–9. doi: 10.1073/pnas.0510577103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liao M-J, Zhang CC, Zhou B, Zimonjic DB, Mani SA, Kaba M, et al. Enrichment of a Population of Mammary Gland Cells That Form Mammospheres and Have In Vivo Repopulating Activity. Cancer Res (2007) 67(17):8131–8. doi: 10.1158/0008-5472.CAN-06-4493 [DOI] [PubMed] [Google Scholar]
- 87.Mohanty ST, Cairney CJ, Chantry AD, Madan S, Fernandes JA, Howe SJ, et al. A Small Molecule Modulator of Prion Protein Increases Human Mesenchymal Stem Cell Lifespan, Ex Vivo Expansion, and Engraftment to Bone Marrow in NOD/SCID Mice. Stem Cells (Dayton Ohio) (2012) 30(6):1134–43. doi: 10.1002/stem.1065 [DOI] [PubMed] [Google Scholar]
- 88.Lee YJ, Baskakov IV. The Cellular Form of the Prion Protein Is Involved in Controlling Cell Cycle Dynamics, Self-Renewal, and the Fate of Human Embryonic Stem Cell Differentiation. J Neurochem (2013) 124(3):310–22. doi: 10.1111/j.1471-4159.2012.07913.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ghazi A, Le Corre D, Pilati C, Taieb J, Aparicio T, Didelot A, et al. Prognostic Value of the PrPc-ILK-IDO1 Axis in the Mesenchymal Colorectal Cancer Subtype. Oncoimmunol (2021) 10(1):1940674. doi: 10.1080/2162402x.2021.1940674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang L, Zuo X, Xie K, Wei D. The Role of CD44 and Cancer Stem Cells. Methods Mol Biol (Clifton NJ) (2018) 1692:31–42. doi: 10.1007/978-1-4939-7401-6_3 [DOI] [PubMed] [Google Scholar]
- 91.Corsaro A, Bajetto A, Thellung S, Begani G, Villa V, Nizzari M, et al. Cellular Prion Protein Controls Stem Cell-Like Properties of Human Glioblastoma Tumor-Initiating Cells. Oncotarget (2016) 7(25):38638–57. doi: 10.18632/oncotarget.9575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Heppner FL, Musahl C, Arrighi I, Klein MA, Rülicke T, Oesch B, et al. Prevention of Scrapie Pathogenesis by Transgenic Expression of Anti-Prion Protein Antibodies. Science (2001) 294(5540):178–82. doi: 10.1126/science.1063093 [DOI] [PubMed] [Google Scholar]
- 93.White AR, Enever P, Tayebi M, Mushens R, Linehan J, Brandner S, et al. Monoclonal Antibodies Inhibit Prion Replication and Delay the Development of Prion Disease. Nature (2003) 422(6927):80–3. doi: 10.1038/nature01457 [DOI] [PubMed] [Google Scholar]
- 94.Abdelaziz DH, Thapa S, Brandon J, Maybee J, Vankuppeveld L, McCorkell R, et al. Recombinant Prion Protein Vaccination of Transgenic Elk PrP Mice and Reindeer Overcomes Self-Tolerance and Protects Mice Against Chronic Wasting Disease. J Biol Chem (2018) 293(51):19812–22. doi: 10.1074/jbc.RA118.004810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Minikel EV, Zhao HT, Le J, O’Moore J, Pitstick R, Graffam S, et al. Prion Protein Lowering Is a Disease-Modifying Therapy Across Prion Disease Stages, Strains and Endpoints. Nucleic Acids Res (2020) 48(19):10615–31. doi: 10.1093/nar/gkaa616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Minikel EV, Karczewski KJ, Martin HC, Cummings BB, Whiffin N, Rhodes D, et al. Evaluating Drug Targets Through Human Loss-of-Function Genetic Variation. Nature (2020) 581(7809):459–64. doi: 10.1038/s41586-020-2267-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Das AS, Zou W-Q. Prions: Beyond a Single Protein. Clin Microbiol Rev (2016) 29(3):633–58. doi: 10.1128/CMR.00046-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Prusiner SB. Novel Proteinaceous Infectious Particles Cause Scrapie. Sci (N Y NY) (1982) 216(4542):136–44. doi: 10.1126/science.6801762 [DOI] [PubMed] [Google Scholar]