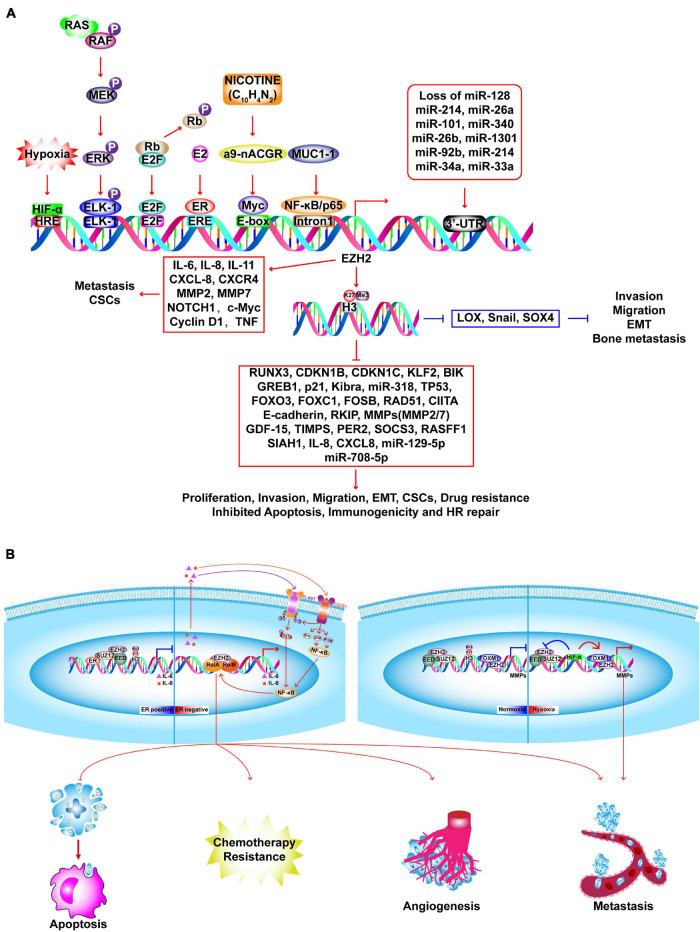
FIGURE 6.
Molecular insights into EZH2-driven BC tumorigenesis. (A) In BC cells, EZH2 expression is upregulated by several factors and loss of microRNAs. Upregulated EZH2 elevate the H3K27me3 and transcriptional repression of several TSGs, such as RUNX3, CDKN1B, CDKN1C, which promote the proliferation, invasion, migration, EMT, CSCs, drug resistance and inhibit apoptosis, immunogenicity and HR repair. EZH2 promotes activation of oncogenes in a PRC2-independent manner, such as IL-6, CXCL-8, NOTCH1, MMP2/7, etc. EZH2 suppresses invasion, migration, EMT, and bone metastasis via inhibiting LOX, SNAIL, and SOX4. (B) EZH2 executes context-dependent activation which can be either dependent or independent on its methyltransferase activity. In ER-positive BC, ER recruits PRC2 complex to the promoter of NF-κB target genes (IL-6, IL-8) to inactivate transcription. In ER-negative basal-like BC, EZH2 acts as a co-activator of RelA and RelB to promote the expression of IL-6, IL-8, and IL-11 which in turn activates NF-κB signaling pathway through a positive feedback leading to constitutive activation of these genes and anti-apoptosis, angiogenesis, metastasis, and chemotherapy resistance. In TNBC, upon normoxia, PRC2 inactivates matrix metalloproteinase gene (MMPs) by catalyzing H3K27me3 at the promoter region. Upon hypoxia, HIF1-α inhibits PRC2 activation by repressing protein expression of SUZ12 and EED, leading to functional switching to EZH2/FOXM1-depedent induction of MMPs expression.