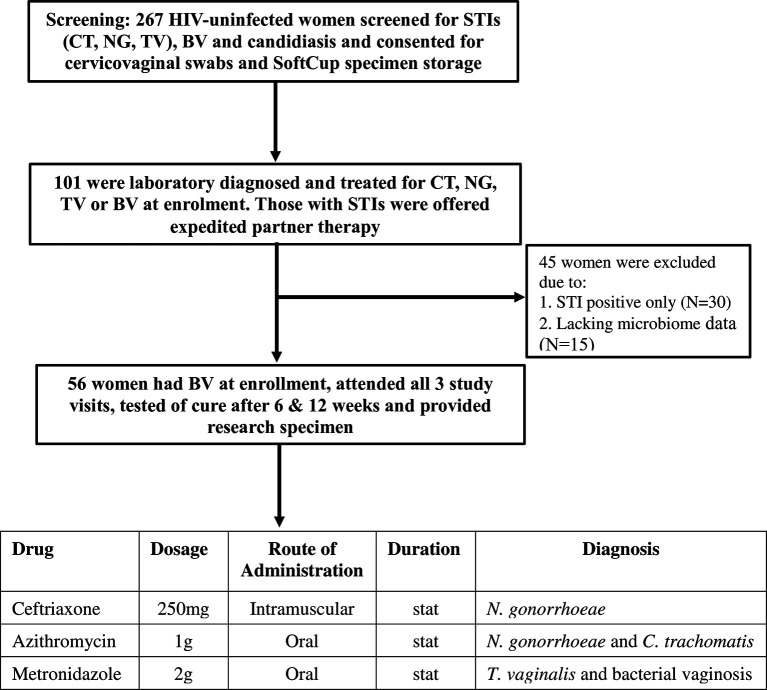
Figure 1.
Screening, enrolment and follow-up of the study participants in the CAPRISA 083 cohort study. Consenting women underwent bacterial vaginosis (BV) and Candida screening and point-of-care (POC) testing for sexually transmitted infections (STI): chlamydia (CT), gonorrhoea (NG) or Trichomoniasis (TV). Women diagnosed with Nugent-BV and any STI were treated, tested for cure at follow-up visits (6 and 12 weeks) and offered expedited partner therapy to deliver to their partners. Mucosal samples were collected at all visits. Women diagnosed with STIs only and/or who did not have vaginal microbiome data were excluded from the study and only those who attended all 3 visits and provided specimens were included.