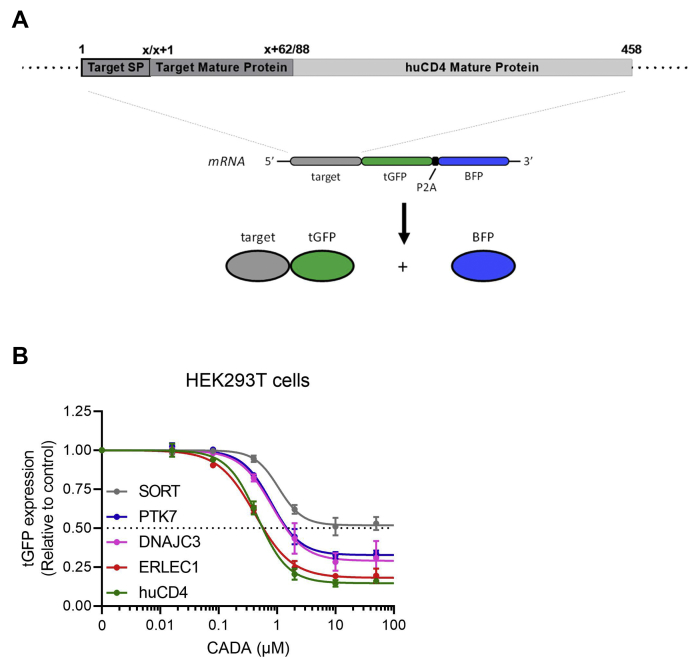
Fig. 3.
SILAC hits differentially respond to CADA in transfected cells.A, schematic representation of the expected mRNA and protein products of the tGFP-P2A-BFP construct. The constructs express huCD4 that is anchored in the plasma membrane via its transmembrane domain and with tGFP at the cytosolic tail. As the SP is cleaved by the ER lumenal signal peptidase during protein biogenesis, the mature huCD4 variants differ only in their N terminus (62 residues). B, four parameter concentration–response curves for CADA of huCD4, SORT, PTK7, ERLEC1, and DNAJC3 cloned in the same tGFP-P2A-BFP plasmid backbone as shown in (A). HEK293T cells were transiently transfected with the tGFP-P2A-BFP constructs and incubated with different CADA concentrations for 24 h. Protein levels in CADA-treated samples are normalized to the DMSO control (set at 1.0). Curves are fitted to data from 3 to 4 replicate experiments. Values are mean ± SD; n ≥ 3. BFP, blue fluorescent protein; CADA, cyclotriazadisulfonamide; DMSO, dimethyl sulfoxide; DNAJC3, DnaJ homolog subfamily C member 3; ER, endoplasmic reticulum; ERLEC1, endoplasmic reticulum lectin 1; HEK293T, human embryonic kidney 293T cells; huCD4, human CD4; PTK7, inactive tyrosine-protein kinase 7; SILAC, stable isotope labeling by amino acids in cell culture; SORT, sortilin; SP, signal peptide; tGFP, turbo GFP.