Fig. 1 |. Structures of mechanically activated ion channels.
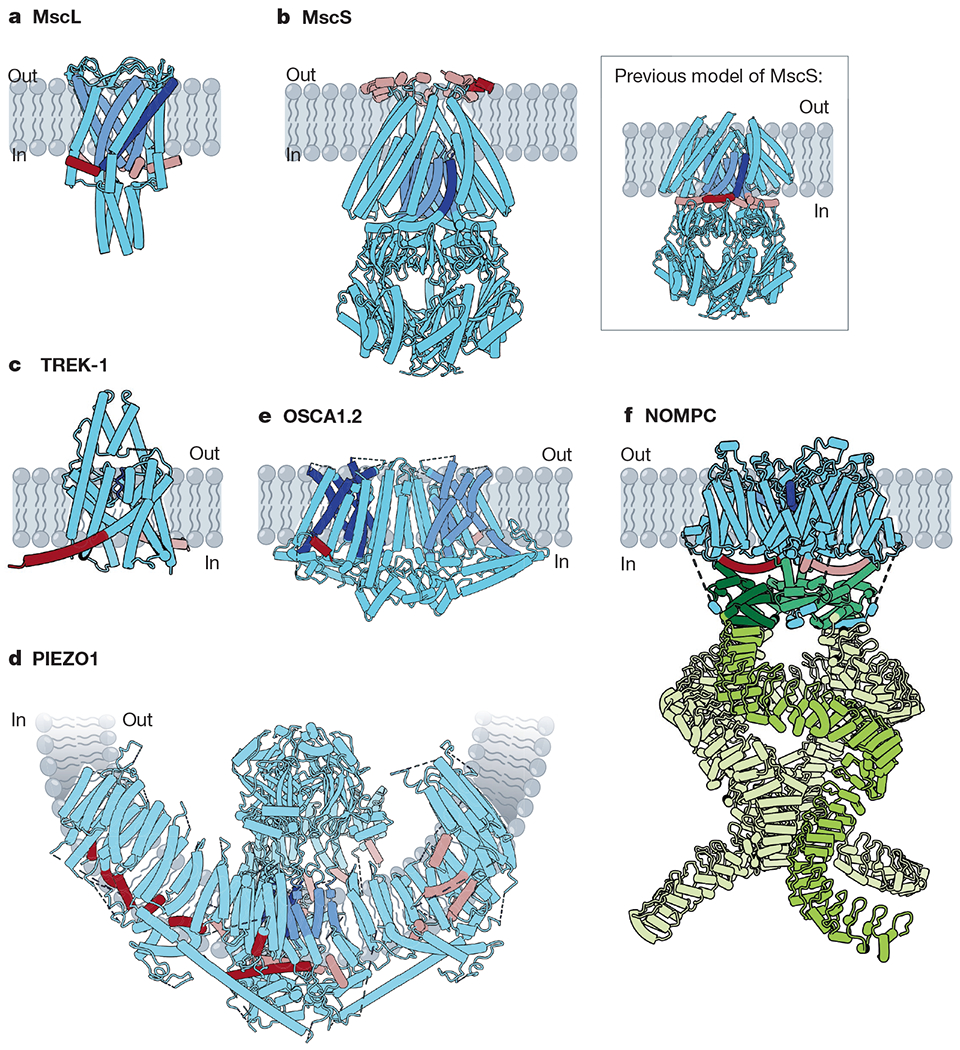
Many mechanically activated channels seem to share a common feature: amphipathic helices (dark red on subunit A, rose on all other subunits) connected directly or indirectly to pore-lining regions (dark blue on subunit A, cornflower on all other subunits). a, Cartoon model of MscL (Protein Data Bank (PDB): 2OAR). Pore-lining TM1 (blue) is connected to the amphipathic S1 helix (red). b, Cartoon models of MscS. Main, a recent structure of MscS in nanodiscs (PDB: 6PWP), with the amphipathic anchor domain (red) sitting on the external membrane leaflet. Inset, the previous model of MscS (PDB: 2OAU), with the pore-lining TM3a helix (blue) completely embedded within the membrane and TM3b (red) predicted to be an amphipathic segment at the cytoplasmic leaflet. c, Cartoon model of TREK-1 (PDB: 6CQ6). The pore domains (blue) are gated by a C-type mechanism61. The amphipathic C-tail (red) extends below the M4 helix. d, Cartoon model of PIEZO1 (PDB: 5Z10). Beneath the extracellular cap, two TM helices from each subunit line the pore (blue). In the domain-swapped blades, several amphipathic helices (red) line the cytoplasmic leaflet. e, Cartoon model of OSCA1.2 (PDB: 6MGV). Five helices (blue) line each of the two putative pores of OSCA1.2 and an amphipathic helix (red) sits on the opposite face of each subunit. f, Cartoon model of NOMPC (PDB: 5VK4). Each NOMPC subunit has an amphipathic TRP domain (red), a pore helix (blue) and a large spring-like ankyrin repeat domain (green).
