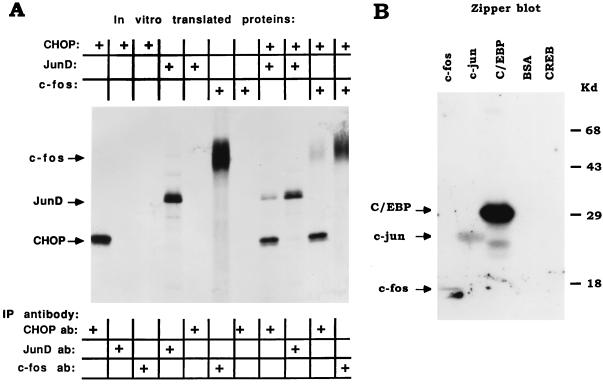
FIG. 5.
CHOP directly interacts with JunD, c-Jun, and c-Fos, as shown by coimmunoprecipitation and zipper blot studies. (A) Coimmunoprecipitation studies. CHOP, JunD, and c-Fos were translated or cotranslated in a reticulocyte lysate system in the presence of [35S]methionine (top). The proteins were immunoprecipitated (IP) with antisera (antibody [ab]) to CHOP, JunD, and c-Fos (bottom). The immunoprecipitated proteins were analyzed by SDS-PAGE and autoradiography. Note that the antiserum to CHOP coimmunoprecipitates both JunD (lane 8) and c-Fos (lane 10). (B) Zipper blot. The proteins designated at the top were bacterially produced recombinant truncated proteins encompassing their respective bZIP DNA-binding and dimerization domain. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane. The membrane was then incubated with a truncated form of CHOP consisting of the bZIP domain that had been labeled with 32P (see Materials and Methods) (38). An autoradiogram of the SDS-PAGE gel shows that CHOP binds to c-Fos and c-Jun, as well as to C/EBPβ (positive control), and not to CREB or bovine serum albumin (BSA) (negative controls).