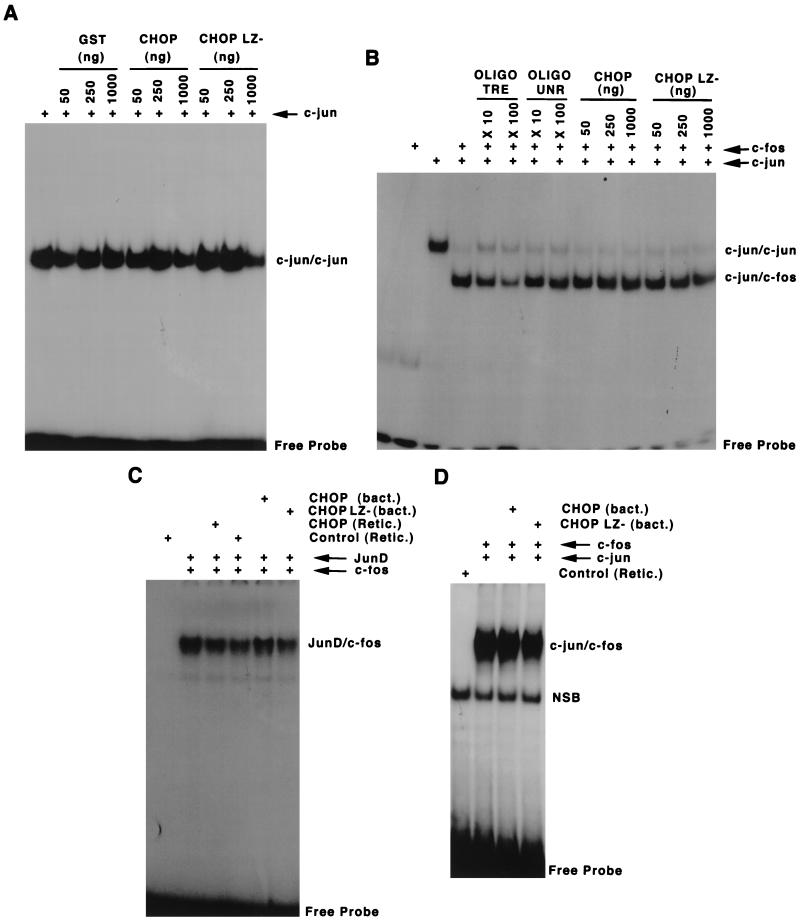
FIG. 7.
CHOP fails to form new stable complexes with AP-1 factors on a TRE. EMSA with the oligonucleotide containing TRE as a gel shift probe and recombinant or in vitro-translated proteins, c-Jun, c-Fos, JunD, and CHOP or its corresponding deletion mutants were used to test the possibility of direct DNA binding of CHOP–AP-1 complexes. (A) A constant amount of recombinant c-Jun was incubated with increasing amounts of recombinant CHOP, its leucine zipper-minus mutant, and the control GST protein. No additional shifting bands were observed in the presence of CHOP. (B) The sifting pattern produced by c-Jun and c-Fos heterodimers was not changed by the presence of increasing amounts of CHOP. A competitive nonlabeled TRE oligonucleotide, but not an unrelated oligonucleotide (UNR), decreased the formation of c-Jun/c-Fos heterodimers in a dose-dependent manner. (C and D) No new retardation complexes, nor changes in binding affinity, were observed when in vitro-translated JunD and c-Fos (C), or c-Jun and c-Fos (D) full-length proteins were used.