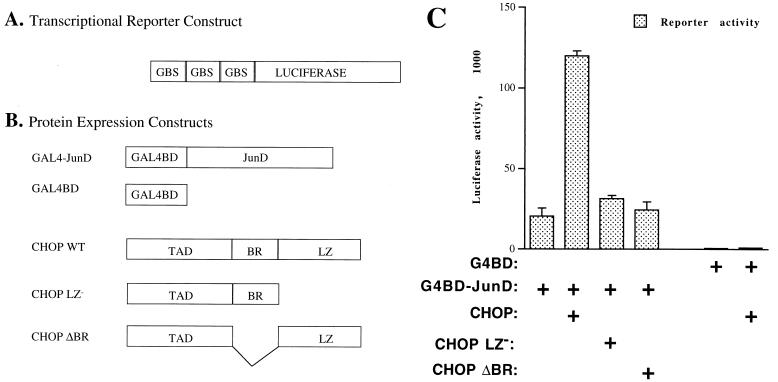
FIG. 8.
Evidence of in vivo physical and functional interactions between CHOP and AP-1 proteins. The mammalian two-hybrid system was used to demonstrate in vivo protein-protein interactions. To determine whether such a protein-protein interaction is also functional, the CHOP transactivation domain was retained instead of being replaced with a heterologous activation domain. (A) Map of the luciferase reporter construct used in these experiments. In this reporter, luciferase expression is driven by three GBS as previously described (49). (B) JunD transactivation of the Gal4 reporter was achieved by fusing the DNA-binding domain of Gal4 (GAL4BD) to JunD to create Gal4-JunD. The maps for the wild-type CHOP (CHOP WT) and the corresponding mutants without the leucine zipper (CHOP LZ−) or the basic region (CHOP ΔBR) are also shown. TAD, transactivation domain. (C) CHOP, but not CHOP LZ− or CHOP ΔBR mutants, enhances the transcriptional activity of Gal4-JunD by severalfold. No direct effect of CHOP was observed on the reporter activity, even when the Gal4 binding domain (G4BD) control was cotransfected along CHOP. These data not only demonstrate that the interaction between CHOP and JunD also occurs in vivo but also indicate that this interaction has a functional relevance.