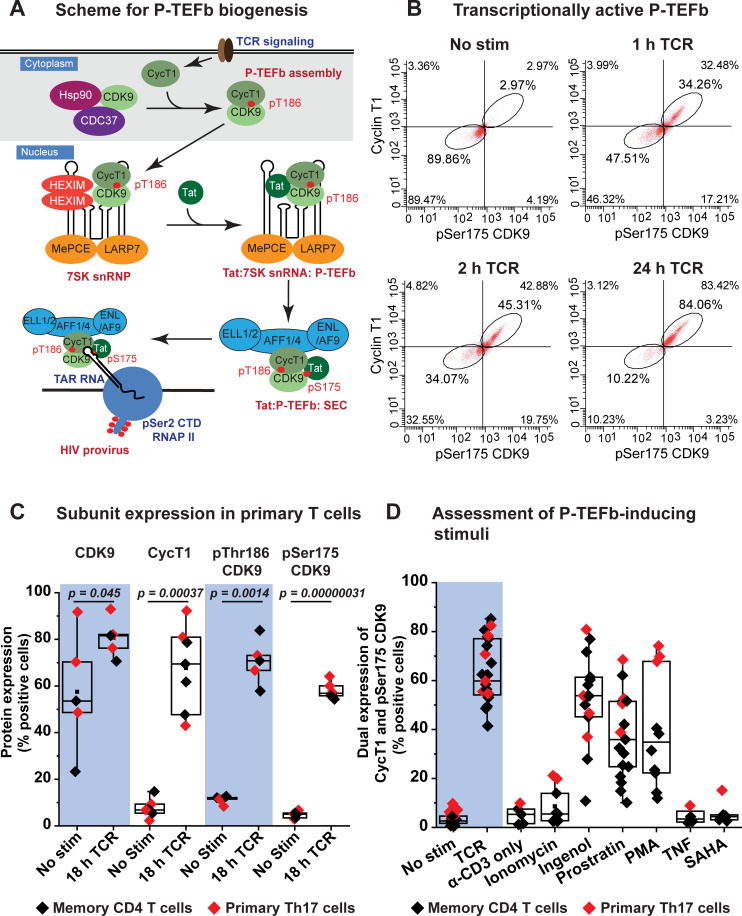
Fig 1. Stimulation of P-TEFb assembly in memory CD4+ T cells by TCR co-stimulation and PKC agonists.
(A) Proposed scheme for the biogenesis of P-TEFb in memory CD4+ T cells and its exchange from 7SK snRNP to assemble the super elongation complex (SEC) on proviral HIV. Posttranscriptional synthesis of cyclin T1 (CycT1) initiated immediately upon TCR co-stimulation serves as a trigger for the heterodimeric assembly of P-TEFb and T-loop phosphorylation of CDK9 kinase at Thr186. Assembled P-TEFb is translocated into the nucleus where it is associated with HEXIM1 and 7SK snRNA to form 7SK snRNP. Exchange of P-TEFb from 7SK snRNP is facilitated by the displacement of HEXIM1 by Tat and also T-loop phosphorylation of CDK9 at Ser175 leading to formation of the super elongation complex (SEC) containing transcriptionally active P-TEFb. This complex is then loaded onto TAR RNA to stimulate proviral transcription elongation. Signaling pathways responsible for the biogenesis of P-TEFb leading up to formation of transcriptionally active P-TEFb will be examined in this study. (B) Assessment of active P-TEFb expression by immunofluorescence flow cytometry as measured by monitoring the co-expression of CycT1 and pSer175 CDK9. (C) Flow cytometry analysis of the intracellular fluorescence immunostaining of total CDK9, cyclin T1 (CycT1), pThr186 CDK9 and pSer175 CDK9 from at least five different experiments performed using either healthy donor-derived memory T cells (black) or in vitro polarized primary Th17 cells (red) that were activated or not for 18 h through the TCR with anti-CD3/anti-CD28 Dynabeads. Statistical significance (p values) was calculated using a two-tailed Student’s t test. (D) Assessment of stimuli capable of inducing P-TEFb expression in primary T cells. The graph shows immunofluorescence flow cytometry measurements of the coordinate induction of CycT1 and pSer175 CDK9 expression in memory T cells (black) derived from at least four different healthy donors or in vitro polarized primary Th17 cells (red). Cells were subjected to 24 h treatment with the indicated stimuli prior to immunofluorescence staining for CycT1 and pSer175 CDK9. The stimuli were used at the following concentrations: a) 1:1 bead-to-cell ratio of anti-CD3/anti-CD28 Dynabeads; b) 1 μg/ml anti-CD3 antibody; c) 1 μg/ml ionomycin; d) 50 nM ingenol; e) 1 μM prostratin; f) 50 ng/ml PMA; g) 10 ng/ml TNF-α; h) 500 nM SAHA.